SP-009-003 How to share performance metrics
What will you learn here?
- What kind of content we can share about performance metrics
- How to answer requests from customers who want to know about the validation of the device
- What documents should you share and not share
Summary
Sometimes, customers may ask things such as:
What's your scientifically proven accuracy in recognizing different skin conditions? Could you share the statistical data?
Do you have studies including Legit.Health use (example of metrics impact such as increasing adequacy of referral)?
This is to be expected. The fact that they ask means that they are doing their job. And your job is to help them understand how our clinical evidence actually demonstrates how amazing our solution is.
Broadly speaking, there are three types of content you can share with customers, each with specific characteristics, confidentiality level and difficulty.
| Blog post and videos | Scientific publication | Clinical investigation | |
|---|---|---|---|
| Complexity for the reader | Low | High | Medium |
| When to share | Freely | Freely | Confidential |
| Who can see | Anyone | Anyone | High-value leads |
| Is the NDA required? | No | No | Recommended, not mandatory |
Keep in mind 🚨
Before moving forward, there is something very important that you must always keep in mind.
You should only share content with a customer if you think that the content will solve a question they have. The content you share must always be specific to their concern.
It is not only annoying but even harmful, to share content with a customer who did not ask for it. Or even worse, to share content that does not address their concern. Imagine a customer asks about increasing patient satisfaction, and you share evidence about reducing referrals. This is very, very dangerous to the survival of the project.
Obviously, it is difficult for everyone to stay updated on the clinical research. So feel free to ask the technical team what content will be best suited for a customer. But to do that, you should provide clear information about what the customer wants.
Blog posts and videos
We seldom publish content on our website that is either a compendium or a simplification of our scientific publications. For example, there is a post in our website called Has Legit.Health taken part in any clinical validation? that lists and contains links to some of our published research.
Likewise, we may produce some video content that explains technical concepts. These can be found on the company's YouTube channel, although you may need to be logged in to see the videos because some of them are not searchable.
Here are some of the videos, but keep in mind that this list is not updated and you may be missing very cool content.
How to share
The best way to share them is to send the customer a link to the video, and ideally, to the exact moment of the video where the interesting part is.
Here's an example of an email sent to a customer:
Hi Michael,
I have something useful for you and your team. Last month we gave a talk with Diego Herrera (Almirall) at an event in Barcelona, and since we published that talk we have seen an increase in pharma companies reaching out and asking about our solution for clinical research. Even asking if we provide training for clinical research coordinators.
Here's the video 👇. For your convenience, I've added links to the key moments:
- 08:33 Almirall explains problems with clinical trials
- 10:12 Almirall explains opportunities with digital clinical trials
- 12:02 Legit.Health explains how the features solve the problems
I believe the video lays out very well our value proposition - and is voiced by a client, so it's quite reliable and validated. I find it very clear, and hopefully, it can help the commercialisation efforts.
Scientific publication
This refers to high-quality peer-reviewed scientific content regarding the most innovative aspects of our technology.
This kind of content is useful, mostly, to our competitors, because it contains an explanation about how to replicate our development process. However, it also shows our commitment to publishing science and it speaks positively of our degree of innovativeness. It also shows that we work with many doctors, who are co-authors of the publications.
Where to find
Scientific publications are published online, so you can find them by looking in Google if you know what you are looking for.
Otherwise, the best way to find them is this QMS. More specifically, R-TF-015-008 Clinical development plan_2023_002. There, you will find a list of the publications, as well as the downloadable PDF and a link to the journal where it was published.
How to share
The best way to share them is to send the customer a link to the journal.
Here's an example of an email sent to a customer:
Dear Stanley,
Regarding your question about how reliable the tool is in the quantification of erythema, feel free to check out this peer-reviewed publication: Automatic SCOring of Atopic Dermatitis.
As you can see, the reliability of the measurement of erythema of our device is higher than that of the clinicians.
Dear Pam,
During our last meeting, you had some questions about the algorithms that check image quality. We actually have this tech published in the Journal of the American Academy of Dermatology: Dermatology Image Quality Assessment (DIQA): Artificial intelligence to ensure the clinical utility of images for remote consultations and clinical trials.
I encourage you to give it a read and let me know if you have any questions.
Clinical investigation
This refers to the clinical investigation that we carry out with hospitals to demonstrate the performance, the clinical benefits and the patient safety of our device. These documents contain information that is sensible and should be shared with discretion. However, they are pretty powerful documents because they addess the performance and explain the claims of the device.
Where to find
Clinical investigations are not published online. The only way to find them is this QMS.
More specifically, in the section Investigation. There, you will find a list of the clinical investigations. For instance:
- R-TF-015-006 Clinical investigation report LEGIT_COVIDX_EVCDAO_2022
- R-TF-015-006 Clinical investigation report LEGIT_MC_EVCDAO_2019
- R-TF-015-006 Clinical investigation report LEGIT.HEALTH_DAO_Derivación_O_2022
The clinical investigation reports are quite long. The most important part is called Clinical Performance, Efficacy, and Safety, which contains a summary of the report.
How to share
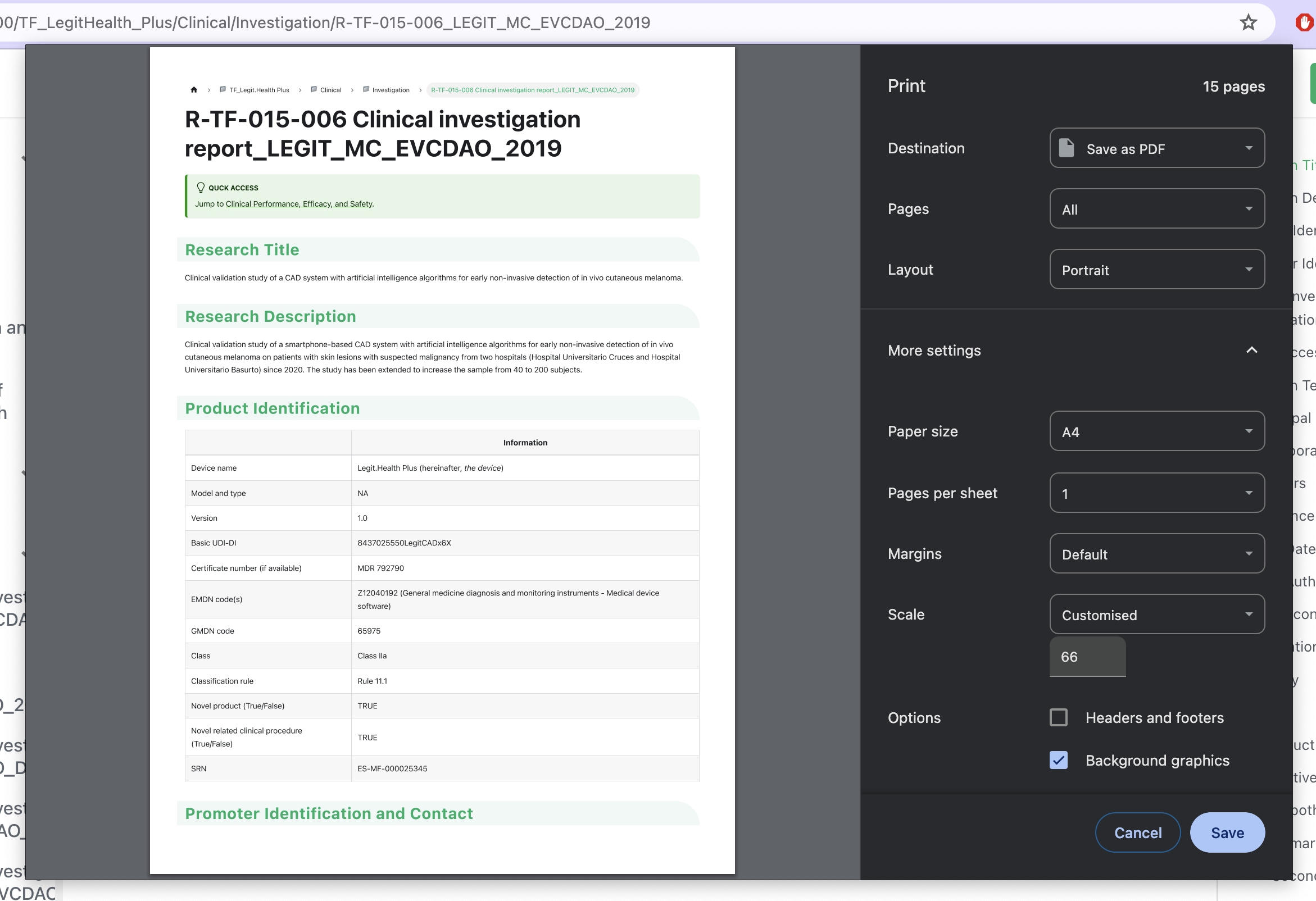
You must open each document in the QMS, and hit cmd + p, which opens the printing dialog. There, select Save as PDF. Then, you share the PDF with the customer.
When creating the PDF, follow these tips:
- Set the zoom to 66% to make the document more readable
- Do not select the option
Headers and footers - Select the option
Background graphic
As this image explains:
Here's an example of an email sent to a customer:
Dear Dwight,
In your email, you asked about studies including Legit.Health use (example of metrics impact such as increasing adequacy of referral). I am attaching to this email some of our clinical investigations.
These are quite important and confidential, so please handle them with care.
The documents you will find attached to this email are:
- R-TF-015-006 Clinical investigation report LEGIT.HEALTH_DAO_Derivación_O_2022.pdf
- R-TF-015-006 Clinical investigation report LEGIT_COVIDX_EVCDAO_2022.pdf
- R-TF-015-006 Clinical investigation report LEGIT_MC_EVCDAO_2019.pdf
The documents are pretty long, but the most important section is called Clinical Performance, Efficacy, and Safety. I would recommend you to start from that section.
Purpose
The purpose of this document is to describe how we share clinical and performance metrics with customers.
Scope
This document applies to the contracts we use during the sales process of all our medical devices, which are software devices.
Responsibilities
Same as the parent procedure, GP-009 Sales.
Associated documents
GP-009 Sales
Document signature meaning
- Author: JD-003 Taig Mac Carthy
- Reviewer: JD-003 Taig Mac Carthy
- Approval: JD-001 Andy Aguilar
ㅤㅤ ㅤ