R-012-010 Device backup verification_2023_001
Evidence
Date
20231017 (YYYYMMDD)
Responsible
JD-005 Alfonso Medela
Evidence
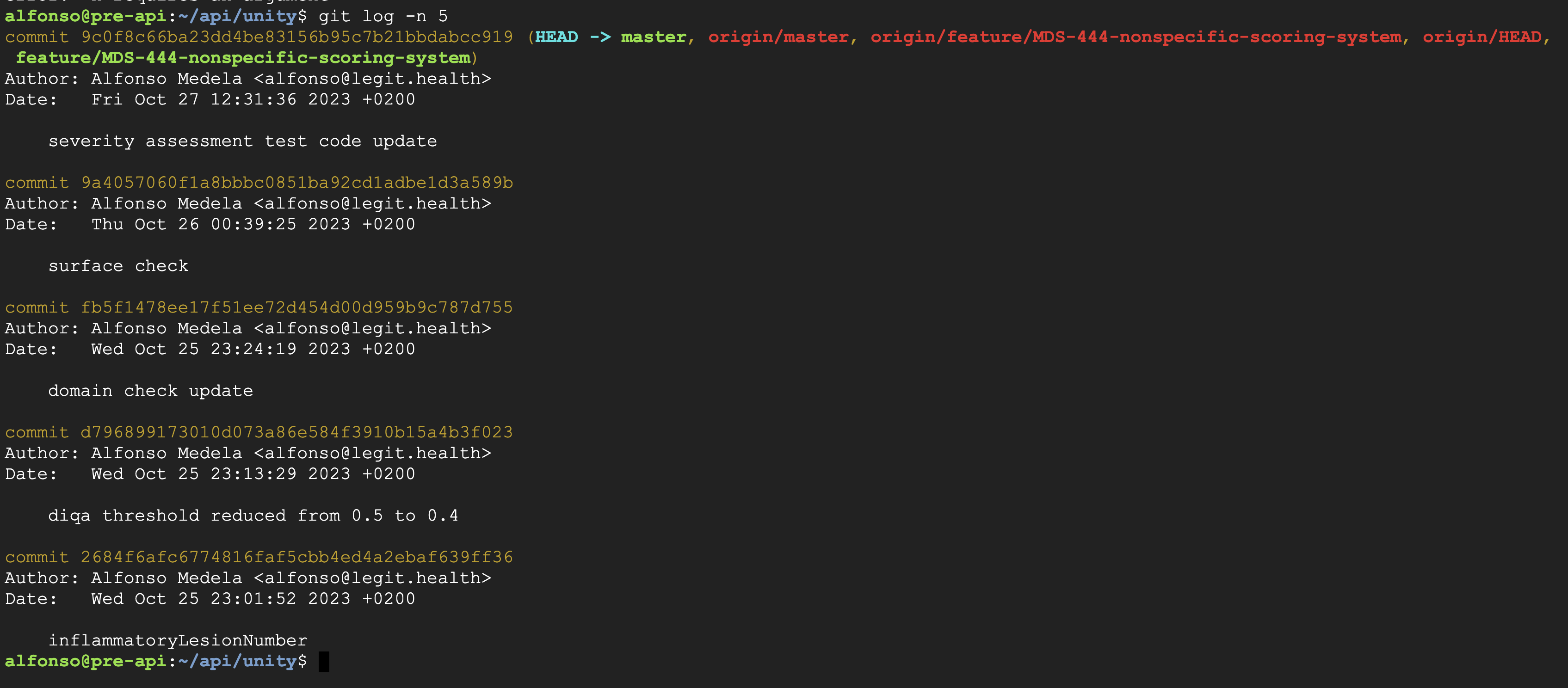
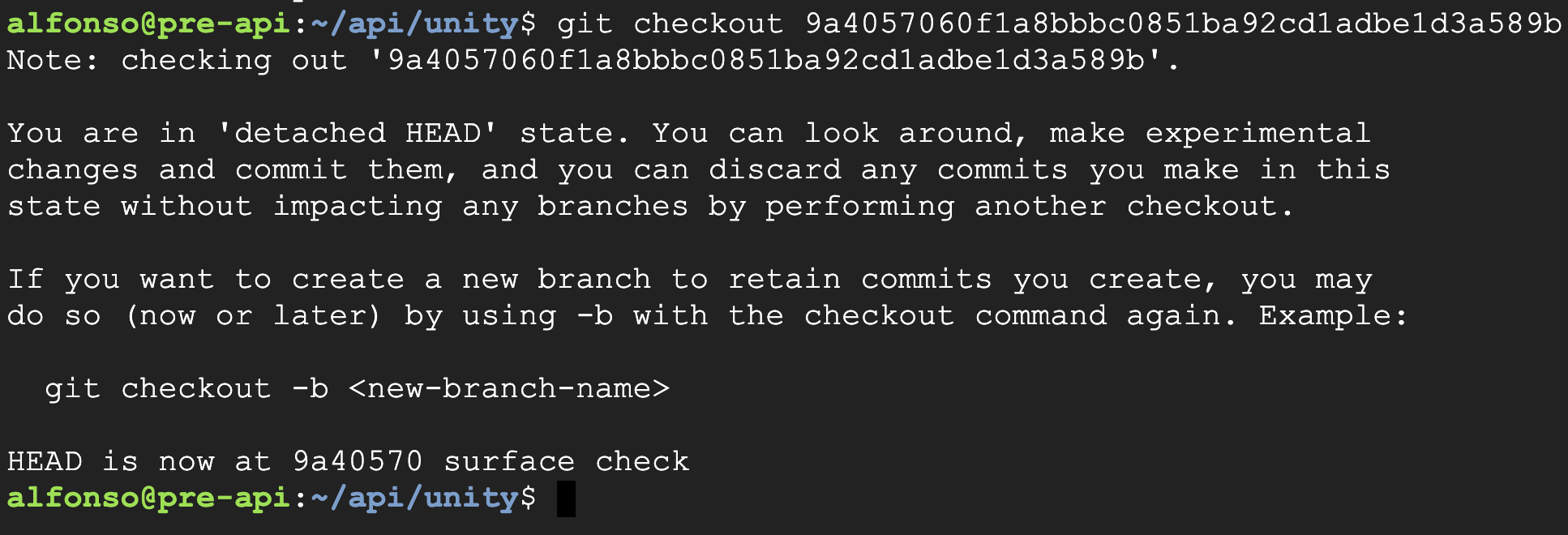
The test was conducted following the procedure GP-012. First, we reverted the git repository to the previous commit (9a4057060f1a8bbbc0851ba92cd1adbe1d3a589b), as indicated in the screenshots below:
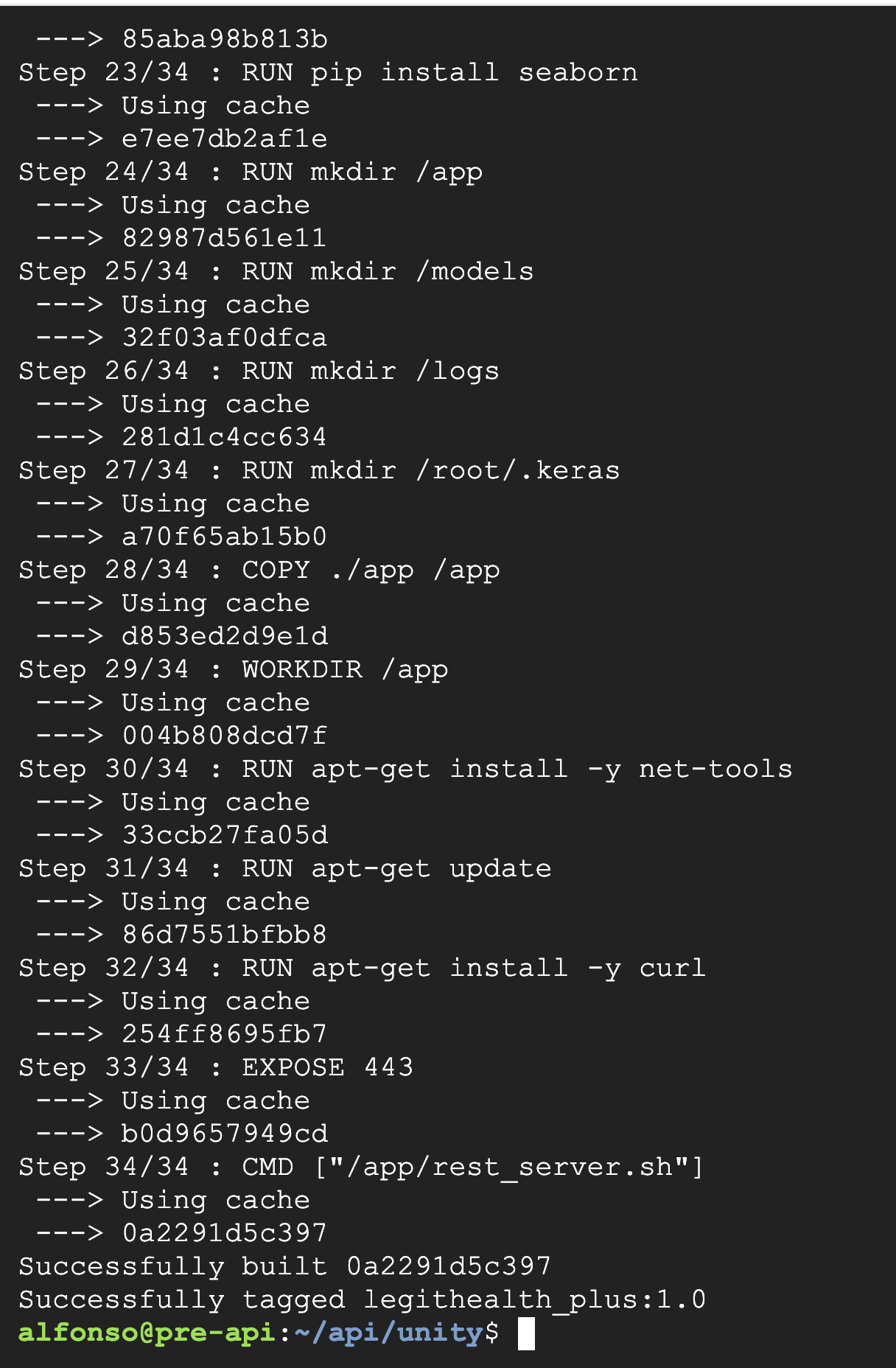
Subsequently, the medical device was compiled. Given that the medical device is dockerized, we executed the docker build command, as illustrated in these screenshots:

Finally, we launched the medical device and ensured it was accessible through the designated server port:


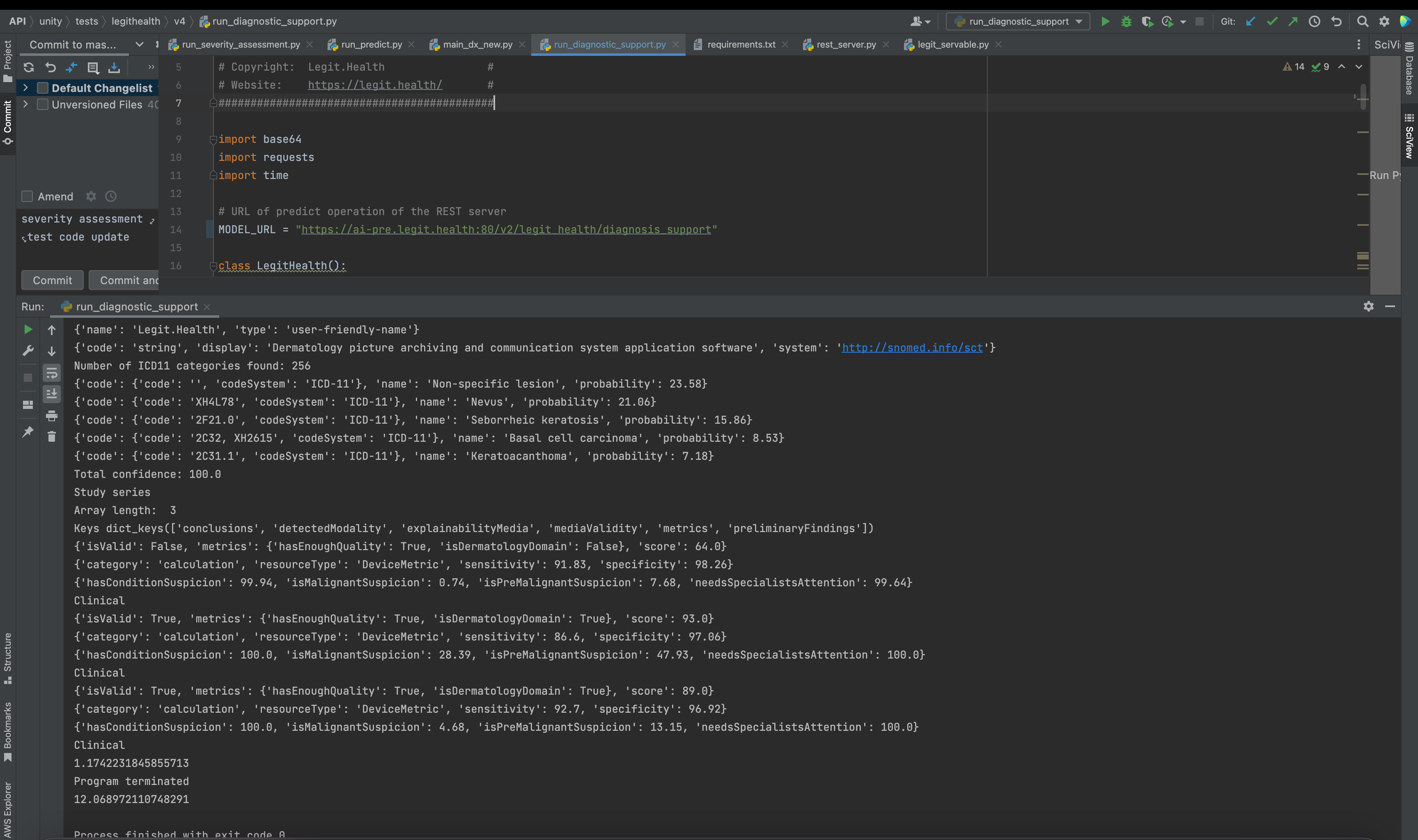
As anticipated, the medical device provided the expected response.
Signature meaning
The signatures for the approval process of this document can be found in the verified commits at the repository for the QMS. As a reference, the team members who are expected to participate in this document and their roles in the approval process, as defined in Annex I Responsibility Matrix of the GP-001, are:
- Author: Team members involved
- Reviewer: JD-003 Design & Development Manager, JD-004 Quality Manager & PRRC
- Approver: JD-001 General Manager