R-019-001 Software validation report_HubSpot_2024
Scope
The aim is to gather additional requirements and configuration specifications not encompassed within the application, together with their respective validations. This ensures adherence to both our internal requirements and those imposed by regulatory bodies. This involves detailing specifications and criteria which are external to the application but fundamental for ensuring our outputs align with all requisite standards and regulations.
Software description
Name
HubSpot
Manufacturer
HubSpot, Inc.
Intended use
The intended use of Hubspot is to have a controlled system to collect and manage all the customers communications, including customers complaints.
Risk-based analysis
The software is used as a controlled system to register the customers complaints. Its failure to perform as intended should not result in a quality problem that foreseeably leads to compromised safety, as we have established a process to manage and follow up with complaints, including the notification of any accident or incident to the National Competent Authorities. As such, the software does not pose a high process risk.
Requirements and design specification
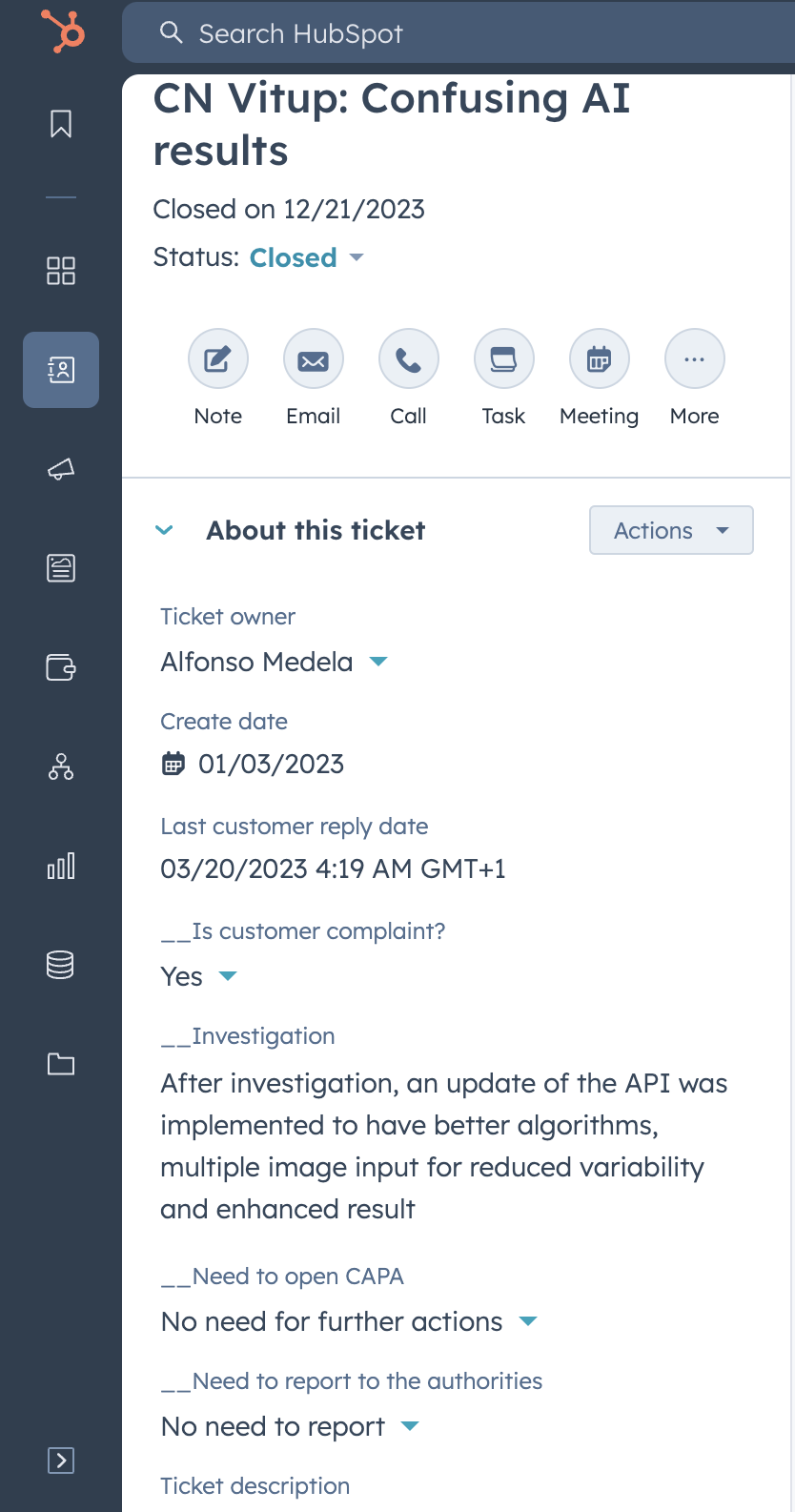
Requirement 01: Users provide the information specified at the GP-014 Feedback and complaints procedure to record if the communication corresponds to a complaint, and the steps to follow.
Assurance activities and test plan
In addition to the tests and checks designed to ensure the configuration complies with the established requirements, we have performed an assessment of the system capability (see R-002-007 Process validation card 2023_002) and a supplier evaluation (see R-010-001 Suppliers evaluation).
| ID | Test description | Acceptance criteria | Requirement tested |
|---|---|---|---|
| Test 01 | Verification that the users can provide all the information required at the GP-014 Feedback and complaints prodedure | The template is correct | Requirement 01 |
Test Results and deviations detected
Test 01
- Result: Pass
- Deviation: No deviations found
Design review
| Result | |
|---|---|
| Have the appropriate tasks and expected results, outputs, or products been established for each software life cycle activity? | TRUE |
| Do the tasks and expected results, outputs, or products of each software life cycle activity: | |
| Comply with the requirements of other software life cycle activities in terms of correctness, completeness, consistency, and accuracy? | TRUE |
| Satisfy the standards, practices, and conventions of that activity? | TRUE |
| Establish a proper basis for initiating tasks for the next software life cycle activity? | TRUE |
Conclusion
No errors were observed in the fields destined for filling the required information. Incorrectly inputting text into the fields is immediately visible and does not impact the safety of the device.
Signature meaning
The signatures for the approval process of this document can be found in the verified commits at the repository for the QMS. As a reference, the team members who are expected to participate in this document and their roles in the approval process, as defined in Annex I Responsibility Matrix of the GP-001, are:
- Author: Team members involved
- Reviewer: JD-003, JD-004
- Approver: JD-001