R-TF-025-004 Summative evaluation protocol
Table of contents
- List of Tables
- List of Figures
- Terminology and Definitions
- Applicable Standards and Guidance
- Internal References
- Introduction
- Description of Intended Device Users, Uses, Use Environments, and Training
- Description of Device User Interface
- Summary of Known Use Problems
- Analysis of Hazards Associated with Use of the Device
- Summary of Preliminary Analyses and Evaluations
- Description and Categorization of Critical and Non-critical Tasks
- Table 5: Critical and Non-Critical Tasks
- Details of Human Factors Validation Testing
- Appendix A: Interview Guide
- Appendix B: Electronic IFU
- Appendix C: List of participants recruited for summative evaluation
List of Tables
- Table 1: Product Interface
- Table 2: Summary of Known Use Problems
- Table 3: Severity Ratings in URRA
- Table 4: Use-related Risk Analysis
- Table 5: Critical and Non-critical Tasks
- Table 6: HCP User Interface for Usability Testing
- Table 7: User Group Details
- Table 8: Participant IDs
- Table 9: Session Overview
- Table 10: Use Scenarios and Descriptions for ITPs
- Table 11: Use Scenarios and Descriptions for HCPs
List of Figures
- Figure 1: Visual Code Studio IDE Interface for ITPs
- Figure 2: Malignant Skin Lesions (Squamous Cell Carcinoma, Melanoma, & Basal Cell Carcinoma)
Terminology and Definitions
- AI: Artificial Intelligence
- API: Application Programming Interface
- CADe: Computer-assisted detection
- CC: Close call
- CFR: Code of Federal Regulations
- DO: Doctor of Osteopathic Medicine
- e.g.,: For example
- EHR: Electronic Health Record
- EMR: Electronic Medical Record
- FDA: Food and Drug Administration
- FHIR: Fast Healthcare Interoperability Resources
- HCP: Healthcare provider
- HD: High definition
- HF: Human factors
- i.e.,: That is
- ICD: International Classification of Diseases
- ICF: Informed consent form
- IDE: Integrated Development Environment
- IFU: Instructions for Use
- IL: Illinois
- IRB: Institutional Review Board
- ITP: Information Technology Professionals
- MD: Doctor of Medicine
- ML: Machine Learning
- OK: Success
- PA: Pennsylvania
- PID: Participant identification code
- RCA: Root cause analysis
- UD: Use difficulty
- UE: Use error
- UI: User Interface
- uFMEA: Use failure mode and effects analysis
- URRA: Use-related risk analysis
- US: United States
Applicable Standards and Guidance
This protocol is written in accordance with the following standards:
- ANSI/AAMI HE75:2009/(R)2018 Human Factors Engineering-Design of Medical Devices
- ANSI/AAMI/IEC 62366-1:2015+AMD1:2020 Medical Devices Part 1: Application of Usability Engineering to Medical Devices
- AAMI/IEC TIR62366-2:2016 Medical Devices Part 2: Guidance on the Application of Usability Engineering to Medical Devices
- ANSI/AAMI/ISO 14971:2019 Medical Devices—Application of risk management to medical devices
- FDA Final Guidance for Industry and FDA Staff: Applying Human Factors and Usability Engineering to Medical Devices (February 3, 2016)
In addition to the standards and guidance documents listed, draft guidance documents were reviewed and considered to understand the current thinking of the FDA on relevant topics.
Internal References
- R-TF-012-014
- REQ*001 The user receives quantifiable data on the intensity of clinical signs * QMS
- REQ*002 The user receives quantifiable data on the count of clinical signs * QMS
- REQ*003 The user receives quantifiable data on the extent of clinical signs * QMS
- REQ*004 The user receives an interpretative distribution representation of possible ICD categories represented in the pixels of the image * QMS
- REQ*005 The user can send requests and get back the output of the device as a response in a secure, efficient and versatile manner * QMS
- REQ*006 The data that users send and receive follows the FHIR healthcare interoperability standard * QMS
- REQ*007 If something does not work, the API returns meaningful information about the error * QMS
- REQ*008 Notify the user if the image does not represent a skin structure * QMS
- REQ*009 Notify the user if the quality of the image is insufficient * QMS
- REQ*010 The device detects if the image is of clinical or dermatoscopic modality * QMS
- REQ*012 Users can easily integrate the device into their system * QMS
Introduction
AI Labs Group (hereafter, the manufacturer) has developed a new software-aided adjunctive diagnostic application programming interface (API) intended to assess clinical atypical cutaneous lesions that are suspicious for skin cancer or other skin conditions. This software-only medical device is intended for use by healthcare information technology professionals (ITPs) to integrate into their hospital systems' electronic medical record (EMR) system, and healthcare practitioners (HCPs) with varying degrees of training in clinical diagnosis and management of skin lesions. Throughout this document, this API will be referred to as the device.
The device is a prescription device that incorporates Artificial Intelligence/Machine Learning (AI/ML) technology, including computer-assisted detection (CADe), which analyzes images or other physical characteristics of a skin lesion. The software provides quantifiable data on the intensity, count, and extent of clinical signs, and an interpretative distribution representation of possible International Classification of Diseases (ICD) classes to aid in determining whether a patient should be referred to a dermatologist.
Design Science will conduct a human factors (HF) validation study to determine if the device and its user interface can be used safely and effectively by all its intended users, for its intended uses, and in its intended use environments. The protocol that follows documents the methodology that will be used.
This validation study will take place in Philadelphia, PA and Evanston, IL from TBD. The study will include simulated-use testing with ITP and HCP participants, who are representative users, to evaluate their use of the product in representative clinical environments.
Description of Intended Device Users, Uses, Use Environments, and Training
Intended Device Users
The intended user population consists of the following distinct user groups:
- IT Professionals (ITPs): IT professionals are responsible for the integration of the medical device into the healthcare organization's EMR system. It is advisable that they have a basic knowledge of Fast Healthcare Interoperability Resources (FHIR) and the output of the device. They are individuals aged 18 years and older and can be from various educational backgrounds. Some of them may have vision, hearing, or dexterity impairments.
- Healthcare Providers (HCPs): Medical professionals (e.g., physicians), who have varying degrees of training in the clinical diagnosis and management of skin lesions, and will utilize the output from the software integration for diagnosis. All of them will have the qualifications and competencies native to their profession, and knowledge on how to take images with smartphones.
Intended Uses
The device is a computational software-only medical device leveraging computer vision algorithms to process images of the epidermis, the dermis and its appendages, among other skin structures. Its principal function is to provide a wide range of clinical data from the analyzed images to assist healthcare practitioners in their clinical evaluations and allow healthcare provider organizations to gather data and improve their workflows. The data generated are intended to aid healthcare practitioners and organizations in their clinical decision-making process. It is not meant to confirm a clinical diagnosis nor replace the role of a dermatologist, but rather to obtain additional information to consider a decision.
The device is indicated for use on images of visible skin structure abnormalities to support the assessment of all diseases of the skin incorporating conditions affecting the epidermis, its appendages (hair, hair follicle, sebaceous glands, apocrine sweat gland apparatus, eccrine sweat gland apparatus and nails) and associated mucous membranes (conjunctival, oral, and genital), the dermis, the cutaneous vasculature, and the subcutaneous tissue (subcutis).
Intended Use Environments
The device is intended to be used in the setting of healthcare organizations and their IT departments, which commonly are situated inside hospitals or other clinical facilities. The device is intended to be integrated into the healthcare organization's system by IT professionals.
The environments are further described as follows:
- IT Office Environment: In the context of healthcare facilities, an IT office is typically designated for hospital IT staff to within the hospital or clinic. The setting is expected to have standard, controlled indoor room temperature, humidity, noise, and lighting conditions. However, during actual use, the intended use environment might vary in ways that impact users' abilities to use the product safely and effectively. ITPs may experience natural distractions from colleagues.
- Clinical Environment: A standard clinical environment is typically designed with a clear layout that has designated areas for patients to be assessed for cutaneous lesions where HCPs can take photos of patients and utilize the device. This setting is also expected to adhere to cleanliness and sanitation standards to prevent infections and include a sufficient inventory of medical supplies. The setting is expected to have standard, controlled indoor room temperature, humidity, noise, and lighting conditions. However, during actual use, the intended use environment might vary in ways that impact users' abilities to use the product safely and effectively. HCPs may experience natural distractions from colleagues and patients. Additionally, the use of gloves can affect HCP's interactions and manual dexterity.
Training
The manufacturer does not expect that users will receive formal training prior to using the device. However, users are expected to review the relevant documentation before use:
- Healthcare Providers (HCPs) must review the Clinical User Manual from Instructions for Use (IFU).
- IT Professionals (ITPs) must review the Installation Manual from Instructions for Use (IFU).
This documentation review is considered a critical task and will be evaluated during the summative evaluation to ensure users can effectively understand and apply the information provided.
Description of Device User Interface
Device User Interface
This software-only medical device consists of a software-aided adjunctive diagnostic application programming interface (API). As such, the user interface of the device software-only medical device consists of the API endpoints, documentation, and data structures, specifically, the electronic instruction for use (IFU) for both user groups as well as API endpoints. These endpoints are used by ITPs for integrating into the hospital IT system. HPCs will then use the user interface of the hospital IT system to interact with the device software-only medical device.
The components of the device API user interface are detailed in Table 1.
| Interface Item | Written Description |
|---|---|
| API Endpoints | URL structures, methods and request structure, response structure, and authentication |
| API Documentation | Electronic IFUs |
| Data Structure | JSON payload formats, including field names and FHIR nomenclature |
Summary of Operating Sequence
The device installation sequence to be conducted by ITPs consists of the following:
- Obtain credentials
- Gain access token
- Build JSON with data
- Send JSON to device
- Receive JSON with device
- Process and store JSON
- Build Report
Refer to the User Guide for a detailed description of the primary operating sequence expected for the device.
For operation by healthcare providers, the device analyzes images taken of lesions and other skin abnormalities and produces a list of potential conditions.
The primary operating sequence consists of the following:
- Take a picture of the lesion with a smart phone
- Image should be close to the lesion, focused, and well lit
- The lesion should be the main item in the photo
- Upload the image to the device client
- Run the analysis
- Review the data output
Refer to the User Guide for a detailed description of the primary operating sequence expected for the device.
Summary of Known Use Problems
The manufacturer has conducted research on known or expected use problems for similar products and product types.
Based on this research, Table 2 describes known use problems pertaining to software-aided adjunctive diagnostic APIs, along with how these are addressed. All of these, along with additional consideration for industry-wide issues, have been identified and documented in the device's risk assessment. These problems are also included in the evaluated during the validation study through observations.
| Type of Use Problem | Description | Applicability to Product | Design Mitigation |
|---|---|---|---|
| User authentication and security | APIs in healthcare settings must adhere to strict security standards. Past experiences have highlighted issues related to complex authentication processes and maintaining compliance with security protocols | Applicable | Implementation of data encryption, robust authentication mechanisms such as OAuth or JWT (REQ_005), monitoring of security threats, cybersecurity info in IFU (REQ_012) |
| Error handling and reporting | Inadequate and/or unclear error messages or lack of real-time error reporting can significantly impact the user's ability to diagnose and fix issues promptly | Applicable | Clear error messages (REQ_007), comprehensive IFU details on correct endpoints (REQ_012), technical support, RESTful API implementation (REQ_005) |
| Interoperability | Ensuring interoperability with various healthcare information systems, including EHR systems, has been a challenge. Compatibility issues can lead to data mismatches or loss, critical in healthcare applications | Applicable | Elastic demand design, constant backups, advanced security/software (REQ_005), REST features for status feedback, error codes, automatic awareness of downtime, support |
| Integration challenges | Difficulties encountered during the integration process, primarily due to compatibility issues with existing systems | Applicable | Clear error messages (REQ_007), comprehensive IFU with correct endpoints (REQ_012), technical support, RESTful API implementation (REQ_005) |
| Insufficient documentation | Inadequate, unclear and/or incomplete guidelines, leading to incorrect implementation and usage errors | Applicable | IFU-provided information (REQ_012), clear error messages (REQ_007), technical support for troubleshooting |
Analysis of Hazards Associated with Use of the Device
The manufacturer has conducted a comprehensive task analysis on the use of the device, which led to a list of potential use errors and a comprehensive use-related risk analysis (URRA). This process involved analyzing known use problems with similar devices, identifying the user interface characteristics related to safety, identifying potential use errors, and identifying known and foreseeable hazards and hazardous situations.
Please refer to R-TF-012-014 for the full use-related risk analysis (URRA).
Table 3 defines the possible severity ratings applied in the URRA.
Table 4 lists the applicable use steps and their severities. It also summarizes the URRA, including tasks, potential use errors, hazards and harms, severities, and risk mitigation measures.
| Severity Classification | Harm Severity Descriptions by Risk Type | Severity Rating |
|---|---|---|
| Catastrophic | Results in patient death. | 5 |
| Critical | Results in permanent impairment or life-threatening injury. | 4 |
| Serious | Results in injury or impairment requiring professional medical intervention. | 3 |
| Minor | Results in temporary injury or impairment not requiring professional medical intervention. | 2 |
| Negligible | Inconvenience or temporary discomfort. | 1 |
ID? | Hazard or Use Error? | Type? | Hazardous Situation or Vulnerability? | Foreseeable sequence of events? | Harm? | Risk or Threat? | Security (CIAA)? | User group? | User task? | Cause Requirement(s)? | Affected Asset, Part or People? | Likelihood (Initial)? | Severity (Initial)? | RPN (Initial)? | Control Opt (ABC)? | Implemented mitigation measures? | Mitigation or Control Requirement(s)? | Responsible? | Verification of implementation of risk control measures? | Severity (Controlled)? | Likelihood (Controlled)? | RPN (Controlled)? | Residual risk evaluation? | Verification of effectiveness of risk control measures? | Benefit-risk analysis? | Risks arise from risk control measures?? | Is risk control complete?? | Overall residual risk acceptability? | Threat Model Ref(s)? | Post-Market Plan Ref(s)? |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| R-HBD | Misrepresentation of magnitude returned by the device | Usability Product | The care provider's system represent a value as if was representing a different magnitude. |
| Misdiagnosis; delay in diagnosis/patient's follow up/treatment | The name of the endpoints of the device do not follow a standard | ITP | Use a stable internet connection | PRS-1XUPRS-5LJ | Managing Organisation | 3 | 3 | 9 | AC | The endpoints of the device follow HL7's FHIR interoperability standard and we developed a integration manual within the IFU that explains the values, as well as a Swagger documentation | PRS-1XUPRS-5LJ | Technical director | IFU verification is recorded at R-TF-001-006 IFU and label validation 2023_001 and in TEST_011 to ensure that they include the information. HL7's FHIR standard compliance is verified at the TEST_013_The data that users send and receive follows the FHIR healthcare interoperability standard. | 3 | 1 | 3 | Acceptable | R-TF-012-015 Summative evaluation report_2024_001 | Not applicable (acceptable risk) | FALSE | TRUE | Acceptable | |||
| R-DAG | Incorrect diagnosis or follow up | Usability Regulatory | The medical device outputs a wrong result |
| This could lead to misdiagnosis; delays in treatment and worsening of the patient's health status. | The interpretive distribution assigns a low probability to the correct ICD class among the potential ICD classes. | HCP | User logs into the system. | PRS-1V6PRS-1XUPRS-5LJPRS-8QJPRS-9J5 | Patient | 4 | 3 | 12 | AC | Information about device outputs are detailed in the IFU. The medical device returns metadata about the output that helps supervising it, such as explainability media and other metrics. The device returns an interpretative distribution representation of possible ICD categories, not just one single condition. AI models undergo retraining using expanded dataset of images. | PRS-1V6PRS-1XUPRS-5LJPRS-8QJPRS-9J5 | Technical director | Process for verification is defined in GP-012 Design, redesign and development. Implementation of device output information in the IFU verified in TEST_011. Verification of the implementation of metadata about the output of the device to help supervising the output: TEST_001, TEST_002, TEST_003. Verification of the implementation of interpretative distribution representation of possible ICD categories verified in TEST_004. | 3 | 2 | 6 | As far as possible | R-TF-012-015 Summative evaluation report_2024_001 R-TF-015-003 Clinical Evaluation Report (sections: Instructions for Use, Associated Design Product Requirement, Valid clinical association of the International Classification of Diseases (ICD) classes, Clinical performance) | Benefits outweigh the risks | FALSE | TRUE | Acceptable | |||
| R-SKK | Incorrect results shown to patient | Usability Cybersecurity Regulatory Artificial Intelligence | The patient see erroneous results. |
| The patient is affected and may suffer anxiety or delays visiting the HCP and their consequent treatment; worsening their health status. | The interpretive distribution assigns a low probability to the correct ICD class among the potential ICD classes.,Patient is using the device without the HCP monitoring | IntegrityAvailability | HCP | User takes a photo of the patient's lesion. | PRS-1V6PRS-1XUPRS-4QWPRS-5LJPRS-8QJPRS-9J5 | Patient | 4 | 3 | 12 | AC | Information about device outputs are detailed in the IFU. The medical device returns metadata about the output that helps supervising it, such as explainability media and other metrics. The device returns an interpretative distribution representation of possible ICD categories, not just one single condition. AI models undergo retarining using expanded dataset of images. | PRS-1V6PRS-1XUPRS-4QWPRS-5LJPRS-8QJPRS-9J5 | Technical director | Process for verification is defined in GP-012 Design, redesign and development. Implementation of device output information in the IFU verified in TEST_011. Verification of the implementation of metadata about the output of the device to help supervising the output: TEST_001, TEST_002, TEST_003. Verification of the implementation of interpretative distribution representation of possible ICD categories verified in TEST_004. | 3 | 1 | 3 | Acceptable | R-TF-012-015 Summative evaluation report_2024_001 R-TF-015-003 Clinical Evaluation Report (sections: Instructions for Use, Associated Design Product Requirement, Valid clinical association of the International Classification of Diseases (ICD) classes, Clinical performance) | Not applicable (acceptable risk) | FALSE | TRUE | Acceptable | T-024-006-AML-001T-024-006-DAT-002 | T-024-007-AUD-001T-024-007-CVE-002 |
| R-E7Z | Inaccessible skin areas | Usability | The device cannot analyse certain skin areas |
| Misdiagnosis; delays in treatment and worsening of the patient's health status. | Inability to access or take a picture of the skin structure due to its location in an unreachable body site and lack of aid in the process | HCP | User takes a photo of the patient's lesion. | PRS-1V6PRS-1XUPRS-5LJPRS-7XK | Patient | 3 | 3 | 9 | AC | A requirement of the device defines the creation of a processor whose purpose is to ensure that the image have enough quality. In other words, an algorithm, similar to the ones used to classify diseases, is used to check the validity of the image and provides an image quality score. The device returns meaningful messages to the users about the quality score of the images. This allows care providers to re-take a photo. The IFU contain the `How to take pictures` section with recommendation on how to take pictures with high quality plus in the Contraindications section of the IFU we state the following: We advise the user not to use the device if skin structures are not accessible by a camera, such as being located in a skin fold or is otherwise covered. We also offer training to the users to optimize the imaging process so that it is optimal for the device's operation. | PRS-1V6PRS-1XUPRS-5LJPRS-7XKPRS-9F2 | Technical director | TEST_009_Notify the user if the quality of the image is insufficient TEST_007_If something does not work, the API returns meaningful information about the error TEST_011_We facilitate the integration of the device into the users' system R-TF-001-006 IFU and label validation | 3 | 1 | 3 | Acceptable | R-TF-015-003 Clinical Evaluation Report (sections: Associated Design Product Requirement, Associated Design Verification Test, Clinical performance) R-TF-012-015 Summative evaluation report_2024_001 | Not applicable (acceptable risk) | FALSE | TRUE | Acceptable | |||
| R-TA9 | Inadequate camera usage or settings | Product Usability | Poor image quality due to inadequate resolution, lighting, focus or camera settings |
| Misdiagnosis; delays in proper treatment and worsening of the patient's health status. | Inadequate image processing algorithms | ITP | Authenticate in the API | PRS-1V6PRS-1XUPRS-2ZBPRS-3YHPRS-5LJPRS-7XK | Managing Organisation | 4 | 3 | 12 | AC | A requirement of the device defines the creation of a processor whose purpose is to ensure that the image have enough quality. In other words, an algorithm, similar to the ones used to classify diseases, is used to check the validity of the image and provides an image quality score. The device returns meaningful messages about the quality score of the images. This allows care providers to re-take a photo. The IFU contain a dedicated section on how to take pictures (section name: `How to take pictures`) and technical specifications of the camera (section: `Technical specifications`) We also offer training to the users to optimize the imaging process so that it is optimal for the device's operation. | PRS-1V6PRS-1XUPRS-2ZBPRS-3YHPRS-5LJPRS-7XK | Technical director | Verification is defined in TEST_009_Notify the user if the quality of the image is insufficient. TEST_007 verifies REQ_007. TEST_011 and R-TF-001-006 IFU and label validation verify REQ_012 | 3 | 1 | 3 | Acceptable | R-TF-015-003 Clinical Evaluation Report (sections: Validation and testing of machine learning models, Post-market Clinical investigations (with focus on clinical studies whose primary endpoint is improving diagnostic accuracy)) R-TF-012-015 Summative evaluation report_2024_001 | Not applicable (acceptable risk) | FALSE | TRUE | Acceptable | |||
| R-UI5 | Inadequate instructions for use: product information for cybersecurity is not included in the IFU | Usability Cybersecurity Regulatory | Presence of vulnerabilities that may compromise the integrity of the system and patient data |
| Unauthorized access to sensitive patient information; incorrect diagnosis; loss of trust | Inadequate information provided by the manufacturer | ConfidentialityIntegrityAvailabilityAuthenticity | ITP | Authenticate in the API | PRS-1V6PRS-1XUPRS-2KQPRS-7Z8PRS-9F2 | Manufacturer | 4 | 3 | 12 | C | We specify in the IFU the product information for cybersecurity in the section `Security requirements and recommendations` | PRS-1V6PRS-1XUPRS-2KQPRS-7Z8PRS-9F2 | Technical director | IFU verification documented in TEST_011 and in R-TF-001-006 IFU and label validation 2023_001 | 3 | 1 | 3 | Acceptable | R-TF-012-015 Summative evaluation report_2024_001 | Not applicable (acceptable risk) | FALSE | TRUE | Acceptable | T-024-006-DOC-001T-024-006-AUT-004 | T-024-007-EDU-001T-024-007-VUL-004 |
| R-5L4 | Inadequate lighting conditions during image capture | Usability Product | The medical device receives an input that does not have sufficient quality |
| Misdiagnosis; delays in proper treatment and worsening of the patient's health status. | Inadequate image processing algorithms | HCP | User takes a photo of the patient's lesion. | PRS-1V6PRS-1XUPRS-2ZBPRS-5LJPRS-7XK | Patient | 4 | 3 | 12 | AC | A requirement of the device defines the creation of a processor whose purpose is to ensure that the image have enough quality. In other words, an algorithm, similar to the ones used to classify diseases, is used to check the validity of the image and provides an image quality score. The device returns meaningful messages about the quality score of the images. This allows care providers to re-take a photo. The IFU contain a dedicated section on how to take pictures (section name: `How to take pictures`) and technical specifications of the camera (section: `Technical specifications`) We also offer training to the users to optimize the imaging process so that it is optimal for the device's operation. | PRS-1V6PRS-1XUPRS-2ZBPRS-5LJPRS-7XK | Technical director | Verification is defined in TEST_009_Notify the user if the quality of the image is insufficient. TEST_007 verifies REQ_007. TEST_011 and R-TF-001-006 IFU and label validation verify REQ_012 | 3 | 2 | 6 | As far as possible | R-TF-015-003 Clinical Evaluation Report (sections: Validation and testing of machine learning models, Post-market Clinical investigations (with focus on clinical studies whose primary endpoint is improving diagnostic accuracy)) R-TF-012-015 Summative evaluation report_2024_001 | Benefits outweigh the risks | FALSE | TRUE | Acceptable | |||
| R-2S3 | Integration failure or errors | Usability Product | Failure to communicate with other systems |
| Misdiagnosis; delayed treatment; loss of trust in the device | Inadequate information provided by the manufacturer | ITP | Authenticate in the API | PRS-1V6PRS-1XUPRS-5LJ | Managing Organisation | 4 | 3 | 12 | C | We specify the intended user and the required qualification in the IFU Additionally, we include at the IFU the instructions and information required by the ITPs to perform the integration of the device within their system | PRS-1V6PRS-1XUPRS-5LJPRS-9F2 | Technical director | IFU verification is recorded at R-TF-001-006 IFU and label validation 2023_001 and in TEST_011 to ensure that they include the information. | 3 | 1 | 3 | Acceptable | R-TF-012-015 Summative evaluation report_2024_001 | Not applicable (acceptable risk) | FALSE | TRUE | Acceptable | |||
| R-HAX | Incorrect interpretation of device outputs | Usability Regulatory | The HCP validates the wrong skin condition, even if the device outputs the correct result |
| Incorrect or delayed diagnosis; inappropriate treatment or follow-up; loss of confidence in the device | Inadequate information provided by the manufacturer | HCP | User takes a photo of the patient's lesion. | PRS-1XU | Patient | 3 | 3 | 9 | C | The IFU explains the medical device's intended purpose The IFU explain the device's outputs The IFU contain a specific section (`User interface`) in which we explain the minimum requirements for the user interface that the ITP will implement | PRS-1XU | Technical director | IFU verification is recorded at R-TF-001-006 IFU and label validation 2023_001 and in TEST_011 to ensure that they include the information. | 3 | 1 | 3 | Acceptable | R-TF-012-015 Summative evaluation report_2024_001 | Not applicable (acceptable risk) | FALSE | TRUE | Acceptable | |||
| R-O5Y | Complicated instructions for use: the instructions for use are too complicated and more intricate than they need to be | Usability Regulatory | Misinterpretation of IFU |
| User discomfort and dissatisfaction; misdiagnosis; delay in diagnosis/patient's follow up/treatment | Inadequate information provided by the manufacturer, lack of clear regulatory guidance or failure to meet labeling standards | HCP | User takes a photo of the patient's lesion. | PRS-0MCPRS-1XUPRS-3YHPRS-5LJ | HCP | 4 | 3 | 12 | C | IFU has been written according to the applicable regulations: MDR 2017/745 and ISO 15223-1 | PRS-0MCPRS-1XUPRS-3YHPRS-5LJ | Technical director | IFU verification is recorded at R-TF-001-006 IFU and label validation 2023_001 and in TEST_011 to ensure that they include the information. | 3 | 1 | 3 | Acceptable | R-TF-012-015 Summative evaluation report_2024_001 Internal/external audits | Not applicable (acceptable risk) | FALSE | TRUE | Acceptable | |||
| R-UK2 | Inadequate warnings in the IFU | Usability Regulatory | Lack of critical safety information required for the correct use of the device |
| Misdiagnosis; delay in diagnosis/patient's follow up/treatment | Inadequate information provided by the manufacturer, lack of clear regulatory guidance or failure to meet labeling standards | HCP | User takes a photo of the patient's lesion. | PRS-0MCPRS-1XUPRS-3YH | Patient | 3 | 3 | 9 | C | IFU has been written according to the applicable regulations: MDR 2017/745 and ISO 15223-1 | PRS-0MCPRS-1XUPRS-3YH | Technical director | IFU verification is recorded at R-TF-001-006 IFU and label validation 2023_001 and in TEST_011 to ensure that they include the information. | 3 | 1 | 3 | Acceptable | R-TF-012-015 Summative evaluation report_2024_001 Internal/external audits | Not applicable (acceptable risk) | FALSE | TRUE | Acceptable | |||
| R-GTY | Instructions for use are not available at the time of use due to downtime | Usability Regulatory | User cannot consult the IFU |
| User discomfort and dissatisfaction; delays in diagnosis; treatment and follow up | IFU are only electronically available, connectivity issue, server issues | ITP | Authenticate in the API | PRS-1V6PRS-1XUPRS-4QWPRS-5LJ | Managing Organisation | 3 | 2 | 6 | A | If the issue is access to the internet, the use would also not be able to use the device, so there is no risk of using the device without access to the instructions. Furthermore, the IFU can be downloaded by PDF. Moreover, the IFU is hosted on a independent instance to improve the resiliency of the information system, this means that downtime in the device does not imply downtime in the IFU. The device sends messages to the user when there is any problem with the communication between the device and the user end, so the user always receives basic instructions when something is wrong. Furthermore, the procedure SP-001-001 - eIFU management explains the process to fulfil customer's request for paper IFU | PRS-1V6PRS-1XUPRS-4QWPRS-5LJPRS-9F2 | Technical director | The selection of independent instances is performed according to GP-012 Design, redesign and development IFU are verified as registered at the TEST_011_We facilitate the integration of the device into the users' system. Process to provide customers with IFU in paper format is explained in SP-001-001 - eIFU management | 2 | 1 | 2 | Acceptable | R-TF-012-015 Summative evaluation report_2024_001 | Not applicable (acceptable risk) | FALSE | TRUE | Acceptable | |||
| R-109 | Electronic instructions for use are not compatible with different devices | Usability Regulatory | Intended user cannot consult IFU |
| User discomfort and dissatisfaction; delays in diagnosis; treatment and follow up | Electronic IFU are developed in a non-universal platform or technology. | ITP | Authenticate in the API | PRS-0MCPRS-1V6PRS-1XUPRS-5LJPRS-7Z8 | Managing organisation | 3 | 2 | 6 | A | The electronic instructions for use are accessible via a web app that is accessible via any browser with any operating system. The instructions do not contain features, graphics or materials that are not universally accessible. It is also relevant to mention that the electronic access to the IFU is actually our recommended method of interacting with them, due to the intrinsic nature of the device Users can request IFU in paper format | PRS-0MCPRS-1V6PRS-1XUPRS-5LJPRS-7Z8 | Technical director | The selection of independent instances is performed according to GP-012 Design, redesign and development IFU are verified as registered at the TEST_011_We facilitate the integration of the device into the users' system | 2 | 1 | 2 | Acceptable | R-TF-012-015 Summative evaluation report_2024_001 | Not applicable (acceptable risk) | FALSE | TRUE | Acceptable | |||
| R-4Z5 | Lack of version control or traceability | Usability Regulatory | The ITP cannot identify the version of the device being used |
| User discomfort and dissatisfaction; delays in diagnosis; treatment and follow up | Inadequate information provided by the manufacturer | ITP | Authenticate in the API | PRS-1V6PRS-1XUPRS-5LJPRS-9F2 | Managing organisation | 3 | 2 | 6 | AC | We include within one of the requirements defined during the design stage that one of the outputs of the device must be the version being used and this information is included in the IFU | PRS-1V6PRS-1XUPRS-5LJPRS-9F2 | Technical director | IFU are verified as registered at the TEST_011_We facilitate the integration of the device into the users' system | 2 | 1 | 2 | Acceptable | R-TF-012-015 Summative evaluation report_2024_001 | Not applicable (acceptable risk) | FALSE | TRUE | Acceptable | |||
| R-BXD | Insufficient knowledge to display electronic IFU | Usability Regulatory | Fail to properly display the instructions for use |
| User discomfort; dissatisfaction. Misdiagnosis; delays in diagnosis/proper treatment and worsening of the patient's health status. | Lack of information/requirement on how to access eIFU | ITP | Authenticate in the API | PRS-1V6PRS-1XU | Managing organisation | 3 | 3 | 9 | AC | IFU is designed in such a way that it is accessible via a dedicated and secure URL and it is also available in the website. The only requirement for accessing the eIFU is having internet connection. The users can access the IFU via any web browsers with any operations system. Upon user's request, we provide the user with IFU in paper format according to the internal procedure SP-001-001 eIFU management | PRS-1V6PRS-1XUPRS-9F2 | Technical director | IFU verification is recorded at R-TF-001-006 IFU and label validation 2023_001 and in TEST_011 to ensure that they include the information. SP-001-001 eIFU management | 3 | 1 | 3 | Acceptable | R-TF-012-015 Summative evaluation report_2024_001 | Not applicable (acceptable risk) | FALSE | TRUE | Acceptable |
Summary of Preliminary Analyses and Evaluations
The manufacturer conducted various preliminary analyses and evaluations during product development. These helped inform the user interface design so it could be optimized with regards to safety and effectiveness.
Description and Categorization of Critical and Non-critical Tasks
In accordance with IEC 62366-1 §5.5 and the FDA definition of critical tasks (21 CFR 803.3(w)), tasks were evaluated and classified based on the potential for user error to result in harm.
A critical task is a user action which, if performed incorrectly or omitted, could result in serious harm to the patient or user, including compromised medical care.
Serious harm or injury includes:
- Life-threatening situations.
- Permanent impairment of a body function or permanent damage to a body structure.
- Situations requiring medical or surgical intervention to preclude such impairment or damage.
Based on the Use-Related Risk Analysis (URRA) severity ratings, tasks with a severity level of 3 or higher were categorized as critical. Tasks logically sequenced together during device use were grouped into use scenarios for summative usability evaluation.
All critical tasks and supporting non-critical tasks that form part of the natural workflow of each use scenario were included in usability validation testing. Non-critical tasks that do not influence completion of critical workflows are considered out of scope for this evaluation.
Table 5: Critical and Non-Critical Tasks
| Usability Task ID | User Group | Phase | Task | Severity | Classification | Use Scenario(s) Evaluated | Justification |
|---|---|---|---|---|---|---|---|
| ITP-T-01 | ITP | IFU Review | Access and read the Instructions for Use (IFU) | 3 | Critical | ITP Use Scenario 1 | Skipping IFU review may lead to incorrect workflow or endpoint usage; could affect downstream AI data processing. |
| ITP-T-02 | ITP | /login Endpoint Request Handling | Authenticate using valid credentials | 2 | Moderate | ITP Use Scenario 1 | Incorrect credentials prevent API access; however, HCP can still treat patients without AI support. |
| ITP-T-03 | ITP | Response Handling | Receive and store the JSON response | 2 | Moderate | ITP Use Scenario 1 | JSON corruption or incorrect storage may delay integration; HCP can still treat patients without AI support. |
| ITP-T-04 | ITP | /diagnosis-support Endpoint Request Handling | Send request to endpoint | 2 | Moderate | ITP Use Scenario 1 | Wrong endpoint or JSON structure prevents correct AI processing; HCP can still treat patients without AI support. |
| ITP-T-05 | ITP | Response Handling | Receive and store the JSON response | 3 | Critical | ITP Use Scenario 1 | Loss or corruption of data could result in misinterpretation of AI outputs. |
| ITP-T-06 | ITP | Response Handling | Confirm JSON contains expected fields as per IFU | 3 | Critical | ITP Use Scenario 1 | Missing fields may prevent AI from producing correct output. |
| ITP-T-07 | ITP | Version Verification | Verify the version of the device API being integrated | 2 | Moderate | ITP Use Scenario 1 | Using the wrong version may cause incompatibility; HCP can still treat patients without AI support. |
| ITP-T-08 | ITP | Error Handling | Handle and log a 400/500 error response from the API | 3 | Critical | ITP Use Scenario 2 | Failure to catch errors could lead to incomplete or invalid AI data. |
| HCP-T-01 | HCP | IFU Review | Access and read the Instructions for Use (IFU) | 2 | Moderate | HCP Use Scenario 1 to 4 | User skips manual review or misinterprets critical instructions. |
| HCP-T-02 | HCP | Take Pictures | Capture clear, well-focused photo of patient’s lesion | 3 | Critical | HCP Use Scenario 1 and 2 | Poor image quality prevents AI from providing meaningful support. |
| HCP-T-03 | HCP | Authenticate in the System | Log into the system (No Lesion scenario) | 2 | Moderate | HCP Use Scenario 1 and 2 | Wrong credentials prevent system access; HCP can still treat patients without AI support. |
| HCP-T-04 | HCP | Upload Pictures | Upload photos to the Legit.Health Plus client | 3 | Critical | HCP Use Scenario 1 and 2 | Missing upload blocks AI processing; HCP lacks support data. |
| HCP-T-05 | HCP | Read and Interpret Report | Understand report format and data fields | 3 | Critical | HCP Use Scenario 1 and 2 | Misinterpretation may lead to incorrect understanding of AI outputs. |
| HCP-T-06 | HCP | Read and Interpret Report | Accurately identify condition probabilities (ICD categories) | 3 | Critical | HCP Use Scenario 1 and 2 | Errors could result in misunderstanding AI-provided probabilities. |
| HCP-T-07 | HCP | Read and Interpret Report | Correctly interpret quantitative outputs | 3 | Critical | HCP Use Scenario 1 and 2 | Misreading quantitative results may misinform clinical support decisions. |
| HCP-T-08 | HCP | Read and Interpret Report | Recognize that report is an aid and not a definitive diagnosis | 3 | Critical | HCP Use Scenario 1 and 2 | Failure to recognize limitations could lead to over-reliance on AI support. |
| HCP-T-09 | HCP | Knowledge Assessment | After use the device, answer the questions of Knowledge Assessment | 3 | Critical | HCP Use Scenario 3 | Misreading quantitative results may misinform clinical support decisions. |
| HCP-T-10 | HCP | Error Handling | React appropriately to an error message (e.g., upload failed) | 3 | Critical | HCP Use Scenario 4 | Ignoring or misinterpreting system warnings could result in incomplete or invalid analysis. |
Justification of Task Criticality and Risk Assessment
The device is a diagnosis support tool intended to provide AI-based clinical insights. It is not a diagnostic device, so patient care can continue without its use. The criticality of tasks is determined based on their potential impact on the reliability and availability of AI outputs and the resulting influence on HCP decision-making.
Non-Critical ITP Tasks: Tasks such as reviewing the IFU or preparatory steps for integration may delay or reduce the efficiency of tool deployment. While skipping these tasks affects the availability of the tool to HCPs, the HCP can still evaluate and treat patients without AI support, so there is no direct impact on patient care.
ITP Critical Tasks: Tasks that involve sending images and data to the API, processing and storing JSON responses, or generating reports are considered critical. Errors in these tasks could either result in no AI output being available (similar to non-critical tasks) or in incorrect AI output, which could potentially misinform HCP clinical support decisions. These tasks are critical because they directly affect the reliability of the AI support information provided to HCPs, even though patient treatment can still proceed without the tool.
HCP Critical Tasks: Tasks such as capturing images, uploading them, and interpreting AI-generated reports are critical because misreading or misusing AI outputs may misinform clinical support decisions. Other HCP tasks like system login are non-critical: failure prevents AI support but does not prevent patient evaluation or treatment.
This classification reflects the intended use of the device, potential severity of use errors, and the fact that patient care is not blocked if the AI tool is unavailable or misused, aligning with IEC 62366-1 §5.5 and the FDA definition of critical tasks (21 CFR 803.3(w)).
Details of Human Factors Validation Testing
Study Objectives
The goal of this study is to assess whether the device and its user interface, including its instructions for use, can be used safely and effectively by its intended users, for its intended uses, and in its intended use environments. Intended users will simulate a typical application case with the device based on its intended use.
To achieve this goal, this validation usability study will be conducted on TBD, at TBD and consists of the simulated use of the device by representative users. The study will consist of a 60-minute one-on-one (moderator and participant) usability testing session, during which the study moderator will give participants, representing potential users, a series of tasks to perform and questions to answer with the device. Throughout the sessions, the study moderator will observe and record participants' objective performance and subjective feedback and ask follow-up questions regarding use of the device. The study will evaluate all observed use problems (i.e., use errors [UEs], close calls [CCs], and use difficulties [UDs]) as well as their associated root causes, see sections 8.6 Description of Data Collection and Methods and 8.7.1 Description of Root Cause Analysis for a summary of the data collection and evaluation method.
The study results will then be used to determine if the residual risk associated with product use is acceptable. While it is rarely feasible to eliminate all risk, it should be reduced to an acceptable level, where feasible and practical, such that the benefits of product use outweigh any residual risks.
Type of Testing
This Human Factors (HF) study will employ simulated-use testing to evaluate how representative users interact with the product in a natural and independent manner. Users will perform tasks under realistic conditions that mimic intended use, allowing observation of potential use errors and assessment of user interface effectiveness.
Simulated-use testing is considered sufficient for usability validation because it can replicate key aspects of actual product use, including:
- Conditions of use (clinical and IT workflows),
- Product characteristics and behavior,
- Typical environmental factors encountered by intended users.
Study Test Environment
Realism
The study environment is designed to approximate the intended use setting for each user group, while maintaining control for observation and data collection:
-
Clinical Environment: HCPs will use the device in a simulated clinical setting, such as a hospital room or physician's office. The room will be quiet and well-lit, allowing HCPs to perform diagnostic tasks, including taking photographs of patient skin lesions and submitting them to a computer integrated with the device software to generate a report. Natural distractions (e.g., phone rings) will not be artificially removed to reflect real-world conditions.
-
Clinical IT Office Environment: ITPs will use the device in a simulated hospital or clinic IT office. The room will also be quiet and well-lit, allowing ITPs to complete tasks such as integrating the device API into the hospital IT system. Natural distractions present in a typical office environment will not be mitigated.
Study Locations
This study will be conducted at Design Science’s facilities in Philadelphia, PA (dates TBD) and in Evanston, IL (dates TBD).
Study Devices and Materials
The devices used in this study will be production-equivalent units, including the electronic Instructions for Use (eIFU). As described in the "Training" section, the manufacturer does not expect users to receive prior training, in order to assess intuitive use and potential user errors under realistic conditions.
Study Devices and Materials - HCPs
To interact with the API, HCPs will be working with a user interface representative of what they may see during actual use following the integration of the API in their hospital system that they will access via a URL on a computer.
While each hospital system may display a different final user interface once the API is integrated, they will all incorporate the same functional elements that are evaluated in this study, namely the portal to upload images, and the subsequent diagnostic report data, i.e., detected conditions, preliminary analysis (malignancy, sensitivity, specificity), and other related data. While stylistic differences exist between various hospital systems, e.g., color schemes, fonts, arrangement of elements, they will all represent the same functions.
As this software-only medical device API only consists of the functions, the GUI design is outside of the scope of this validation study, and only the functional layer of the interface, i.e., the API interaction, will be assessed. Therefore, testing the API with a generic version of the user interface will be sufficient to evaluate the API. This generic interface is shown in Table 6.
| User Interface Item | Written Description | Image |
|---|---|---|
| Software | Initial screen where HCPs will upload the photo of the lesion | 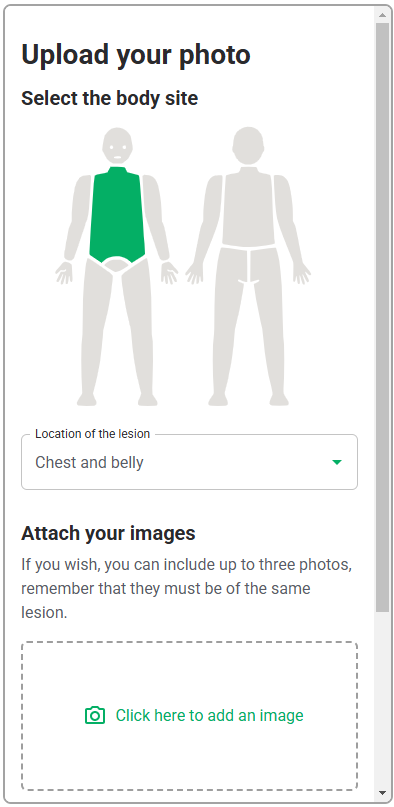 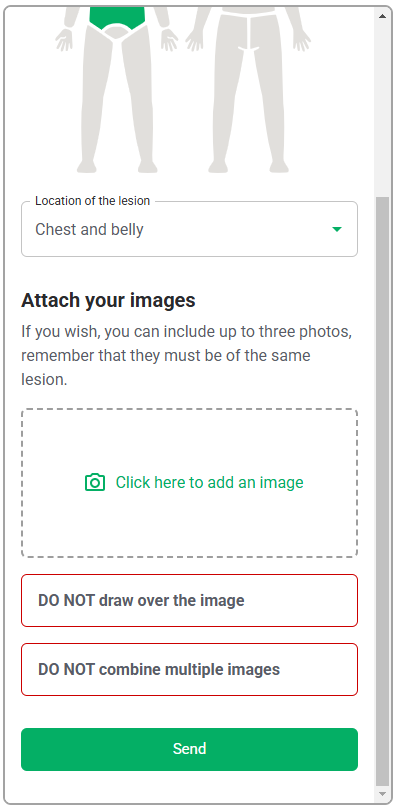 |
| Diagnostic report | Details of the diagnostic report following submitting the photo of lesion | 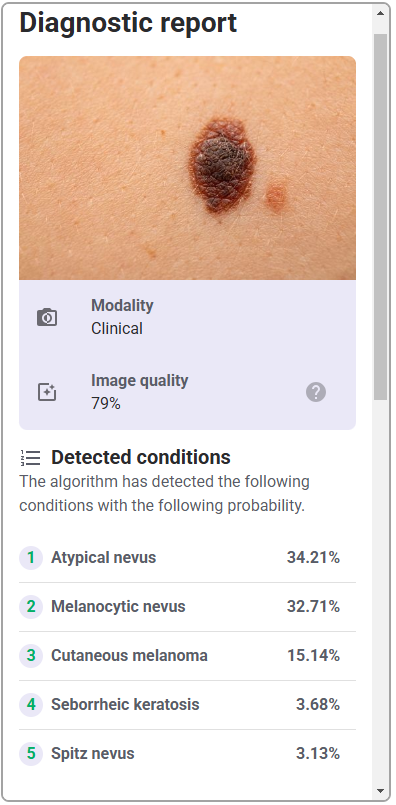 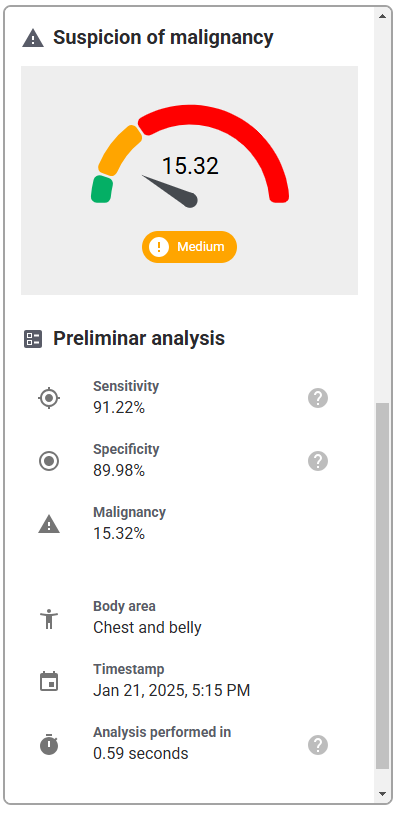 |
Based on section "Description of Device User Interface", the following materials will be present in the study environment to simulate items that HCPs may use during their diagnostic assessment of a patient:
- Smartphone with the capability of taking high quality photos supplied by the manufacturer for usability testing
- Computer with the device software integrated into a representative EHR/EMR system
- Gloves
- High quality digital images of skin lesions supplied by the client for participants to upload to the device
ITPs use Postman to send requests, inspect responses, handle errors, and verify API workflows. Therefore, Postman is sufficient for usability testing. Figure 1 displays the user interface for Postman, a representative API client that ITPs would use in their typical practice.
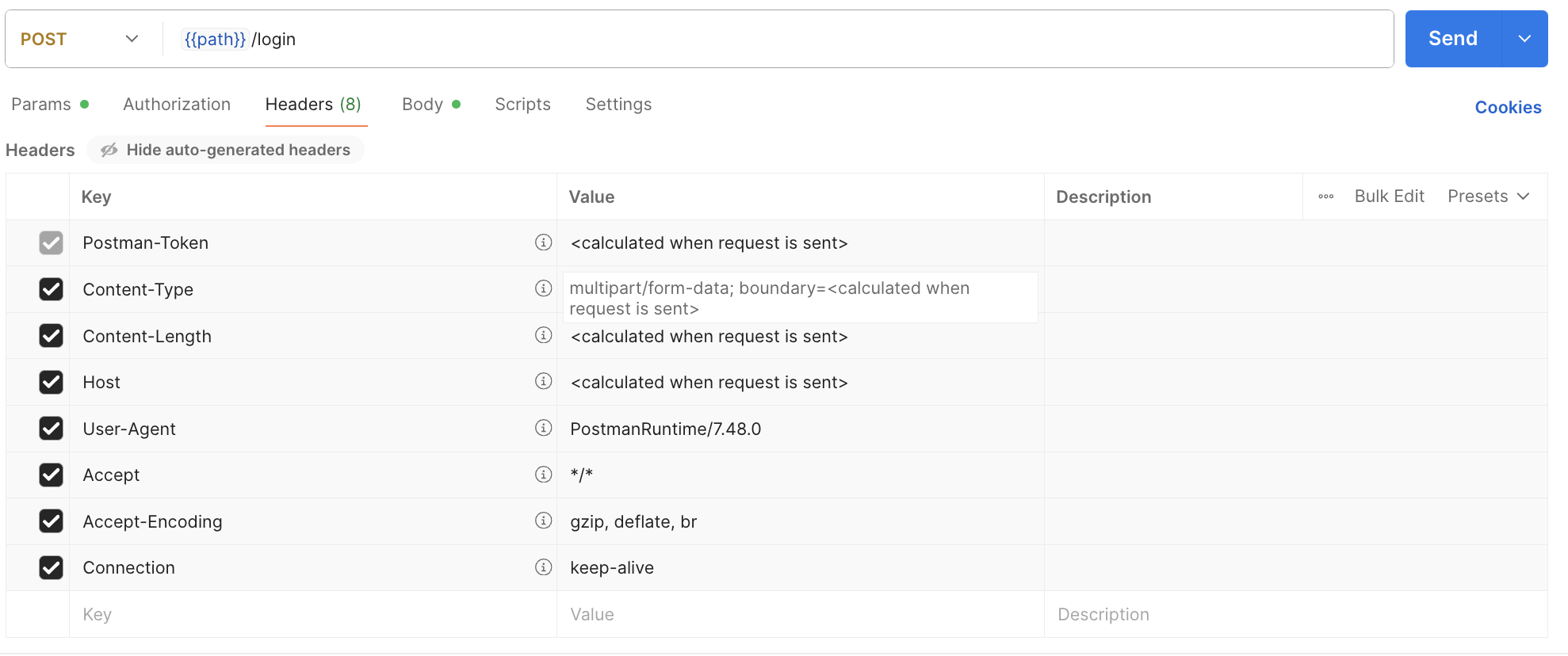
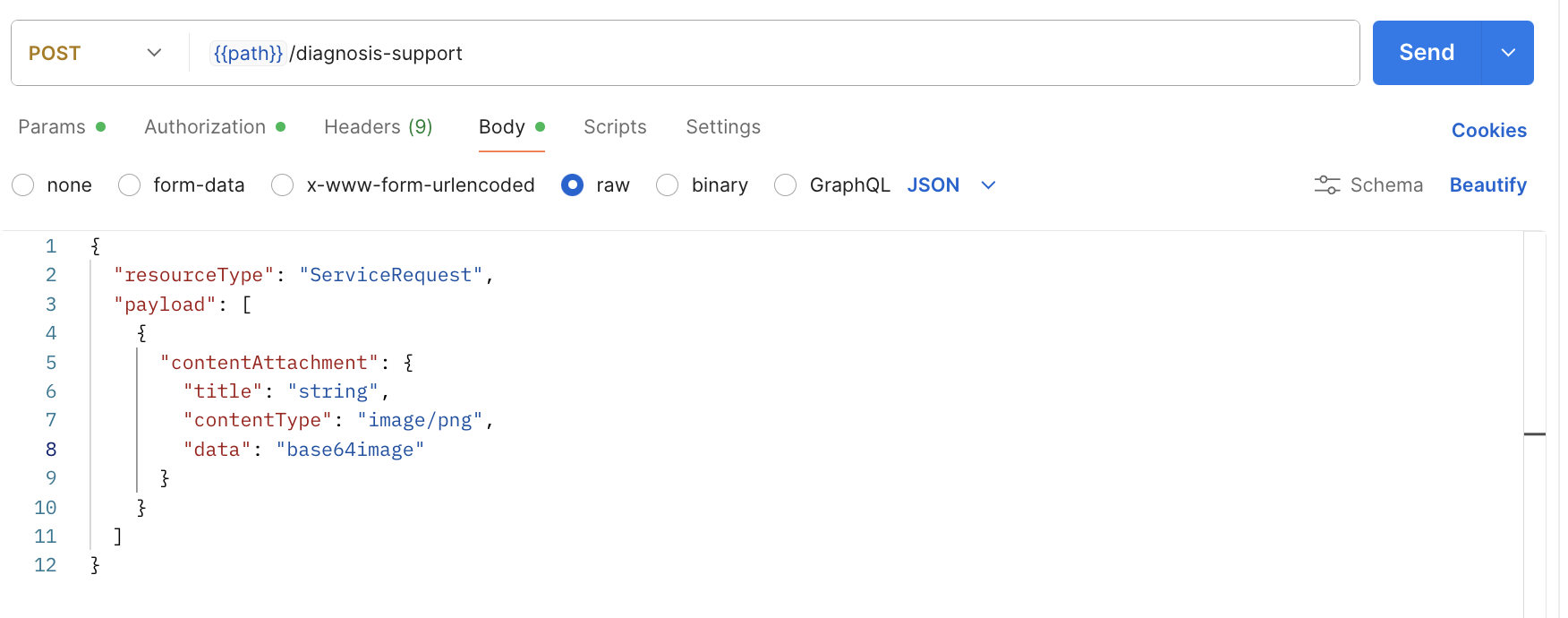
Figure 2: Postman Interface for ITPs. Example of diagnostic support request.
This setup allows participants to interact directly with the API endpoints (authentication, data submission, error handling) in a realistic and controlled environment. Performing full system integration into a CRM or EHR is not required for usability validation under IEC 62366-1, as this would constitute system-level verification rather than user interface validation. The use of Postman sufficiently represents the ITP interaction with the API and enables identification of usability-related issues (e.g., unclear documentation, ambiguous error messages, confusing parameters) that could impact safe and effective integration.
Based on section 3 Description of Device User Interface, the following materials will be present in the study environment to simulate items that ITPs may use to complete their work:
- Computer with a representative EHR/EMR system and Postman installed
- Access credentials (user and password) for accessing device endpoints
- Electronic IFU for reference. Including:
- Installation Manual
- API Documentation
Participants
Number, Type, and Rationale
The study will include 30+6 participants (i.e., 15 IT professionals, and 15 healthcare providers) who are representative of the intended users. There will be an additional 3 participants recruited for each user group to account for potential no-shows, disqualifications, and cancellations; however, testing will end after the fifteenth participant for each user group. Refer to Attachment B-Participant Screener for full inclusion/exclusion criteria.
Table 7 describes the user groups intended user information.
| User Group | # of Participants | Training Status | # of Sessions | Characteristics |
|---|---|---|---|---|
| ITPs | n = 15 + 3 | Untrained | 1 | - Ages 18 years or older - Have experience with FHIR - Have experience integrating services and API into a hospital IT system - At least 1 year of experience working for a hospital IT department |
| HCPs | n = 15 + 3 | Untrained | 1 | - Ages 18 years or older - Licensed physician (MDs, DOs) - Varying degrees of training in clinical diagnosis and management of skin lesions - At least 1 year of experience as an HCP |
Inclusion and Exclusion Criteria
In addition to the defined criteria, to reduce bias and ensure representative users have been selected as participants, all participants must
- not be affiliated with market research, product development, pharmaceutical companies, or medical device companies,
- not have completed any medical market research related to software-aided adjunctive diagnostic devices within the last 3 months,
- bring photo identification to the study,
- have the capacity to understand and sign an informed consent form (ICF),
- be able and willing to participate in a 60-minute session and complete study assessments,
- be willing to be video recorded during the study,
- be able to speak, read, and comprehend English, and
- be a EU|US resident.
Participant Protection
Identity Protection
The study moderator will assign a unique identification code (i.e., participant ID) to each participant as a means of referencing participants, so that the manufacturer is not able to associate participant names or any other unique personal identifiers with the study data.
The letter component of the participant ID corresponds to the user groups as presented in Table 8. The numerical component of the participant ID (ranging from 01-15) refers to the order in which each participant from each user group participated in the study.
| Participant ID | User Group |
|---|---|
| ITP-### | IT Professionals |
| HCP-### | Healthcare Provider |
Institutional Review Board Oversight
This human factors study will not be submitted to an Institutional Review Board (IRB) due to the following reasons:
- Risk to the participants is minimal. Testing is non-invasive and structured in a simulated-use environment.
- There will be no needles, treatment, or drug product present. Participants will be interacting with a software-only device.
- Participants will review and sign an informed consent form (ICF) and business confidentiality agreement providing consent to participate in the evaluation.
Tasks and Scenarios
Session Overview
The structure of each 60-minute testing session is detailed in Table 9.
| Session Phase | Description |
|---|---|
| Informed Consent Review | The study moderator will review the ICF with the participant, ensure that they understand the form, agree to participate, and agree to be recorded. |
| Documentation Review | Participants will review relevant documentation (Clinical User Manual for HCPs, Installation manual for ITPs). Understanding will be verified through targeted questions. |
| Background Questions | The study moderator will ask the participant a series of background questions to confirm that they meet the study inclusion criteria and collect relevant data. |
| Overview | An overall explanation of the testing procedure will be given. |
| ITP Use Scenario 1: Simulated Use Post Scenario Interview (including Root Cause Interview) | Each of the use scenarios will be administered, followed by an interview. |
| Conclusion | Participants will be given a chance to express any additional questions or comments. |
| Session Phase | Description |
|---|---|
| Informed Consent Review | The study moderator will review the ICF with the participant, ensure that they understand the form, agree to participate, and agree to be recorded. |
| Background Questions | The study moderator will ask the participant a series of background questions to confirm that they meet the study inclusion criteria and collect relevant data. |
| Overview | An overall explanation of the testing procedure will be given. |
| HCP Use Scenario 1: Simulated Use: No Lesion Post Scenario Interview (including Root Cause Interview) | Each of the use scenarios will be administered, followed by an interview. |
| HCP Use Scenario 2: Simulated Use: Lesion Post Scenario Interview (including Root Cause Interview) | Each of the use scenarios will be administered, followed by an interview. |
| HCP Use Scenario 3: Knowledge Assessment Post Scenario Interview (including Root Cause Interview) | Each of the use scenarios will be administered, followed by an interview. |
| Conclusion | Participants will be given a chance to express any additional questions or comments. |
Use Scenarios and Tasks
All participants will complete all use scenarios described in Table 10 and Table 11 in that order. Prior to the completion of the use scenarios, the study moderator will introduce participants to the study environment and give a brief introduction to each scenario. Critical tasks must be completed successfully to demonstrate the safety and effectiveness of the device.
During the first scenario, participants will be asked to imagine that they have just received the device and must use it for the very first time, just as they would in a real-life situation.
ITPs will have the electronic IFU containing installation instructions for the device software available to use if they chose so. Also, a computer will be set up with a typical representation of hospital IT systems. This would include an installed EHR/EMR program and a representative IDE. They will be provided with a URL with the device API and will be tasked with integrating it with the hospital IT system on the computer. In order to assess the API, the study moderator will observe participants' behaviors and actions and note any use problems that occur throughout the integration process (e.g., difficulty logging in, managing tokens, understanding error messages etc.). The study computer will enable a screensharing feature that will allow the study team to observe the participant's progress. Furthermore, as participants interact with the API during simulated use, a log that is visible to the manufacturer is automatically generated that tracks their progress to confirm the completion of the assigned tasks. A representative from the manufacturer's team will be monitoring the log during the sessions to assist in identifying and characterizing any observed use problems.
| Use Scenario | Phase | Task | Classification | Success Criteria |
|---|---|---|---|---|
| ITP Use Scenario 1: Simulated Use | Build JSON with data | Enter data | Critical | User does not enter data in incorrect types (e.g., string instead of integer) |
| ITP Use Scenario 1: Simulated Use | Build JSON with data | Enter required fields | Critical | User enters required fields for the request |
| ITP Use Scenario 1: Simulated Use | Build JSON with data | Format the data | Critical | User formats the data correctly, does not include the wrong JSON structure or incorrect field names |
| ITP Use Scenario 1: Simulated Use | Build report | Generate report | Critical | User understands the data format, and the data are displayed consistently. |
| ITP Use Scenario 1: Simulated Use | Build report | Send images to the API | Critical | User codes for images to be sent in a supported format, or correctly encoded in Base64 |
| ITP Use Scenario 1: Simulated Use | Build report | Send required data to the API | Critical | User does not include extraneous data not required for the request |
| ITP Use Scenario 1: Simulated Use | End of Session | Save and document results | Critical | User saves the sandbox results and documents the verification outcome according to the usability protocol. |
| ITP Use Scenario 1: Simulated Use | Get access token | Authenticate in the API | Critical | User inputs correct username, password, or other required authentication details |
| ITP Use Scenario 1: Simulated Use | Get access token | Use a stable internet connection | Critical | User uses a stable internet connection |
| ITP Use Scenario 1: Simulated Use | Get and store JSON response from the device | Extract the data | Critical | User successfully downloads the JSON response file from the device without altering its content. |
| ITP Use Scenario 1: Simulated Use | Receive JSON from device | Process the request | Critical | The user completes the response without corrupting it, misinterpreting the HTTP status, deleting or modifying it, or incorrectly mapping the response data to the clinical workflow or patient records. |
| ITP Use Scenario 1: Simulated Use | Send JSON to device | Input correct HTTP method | Critical | User uses the correct HTTP method |
| ITP Use Scenario 1: Simulated Use | Send JSON to device | Send access token with the request | Critical | User includes the access token in the header. The access token is not expired, and the request is only sent once |
| ITP Use Scenario 1: Simulated Use | Send JSON to device | Send request to the endpoint | Critical | User sends the request to the correct endpoint |
| ITP Use Scenario 1: Simulated Use | Simulated Use | Paste JSON response into internal sandbox tool | Critical | User correctly pastes the JSON response into the designated internal sandbox environment without syntax errors or missing content. |
| ITP Use Scenario 1: Simulated Use | Simulated Use | Run visualization in the sandbox | Critical | User successfully runs the sandbox environment to visualize the data as described in the IFU. |
| ITP Use Scenario 1: Simulated Use | Verification of Output | Verify displayed data | Critical | All response data are displayed correctly and match the expected structure and clinical information as defined in the IFU. |
HCPs will be tasked with using the API through a representative GUI to assist in diagnosing a patient that has come in to assess a skin lesion. To simulate taking photos, the HCP will be asked to take a photo of the hand of a member of the research team such as the study moderator or a second supporting analyst. The HCP will then submit it to the device that has been installed on a computer, running the report, and interpreting the results to see if they are consistent with the lesion. The HCP will be tasked with following this workflow to assess skin that does not bear a malignant lesion using the device only one time. The study moderator will then observe participants' behaviors and actions and note any use problems that occur during the usage of the software.
Following this simulated-use scenario (Scenario 1), a post scenario interview will be performed to better evaluate safe and effective use. To this end, participants will be asked how they felt the scenario went as well as whether they made any mistakes, almost made any mistakes, or had any difficulties. After this self-reflection, the moderator will probe for better understanding and delve into a root cause interview, where open-ended questions will be asked until the underlying cause of each use problem can be identified. This will include participant-reported and moderator-observed use problems from Scenario 1. For some tasks, successful completion may or may not be observable during the simulated-use scenario due to the nature of the task. For example, a task may require the participant to correctly interpret the data produced by the product (e.g., read the list report output). If task performance is not observable, the moderator may follow up in the root cause interview and ask open-ended, naturally worded follow up questions about the task to accurately assess the participant's task performance.
Following this, the HCPs will be asked to complete their workflow once again and will be directed to use the device to produce a report for a malignant skin lesion (Scenario 2). A digital file containing photos of a skin lesion will be provided to HCPs. The HCP will then upload these photos to the device that has been installed on a computer, running the report, and interpreting the results to see if they are consistent with the lesion. The HCP will be tasked with following this workflow to assess a malignant lesion using the device only one time. The study moderator will then observe participants' behaviors and actions and note any use problems that occur during the usage of the software.
For the HCP workflow, some important aspects of use, such as interpreting and understanding the report, cannot be assessed through task performance and instead are assessed through direct questioning of the participant to ascertain their understanding of information. This knowledge is tested by questioning the test participants using open-ended and naturally worded questions (Scenario 3). The knowledge assessment will be administered to determine their understanding of the information in the report output. The malignant skin lesions participants may assess are shown in Figure 2.
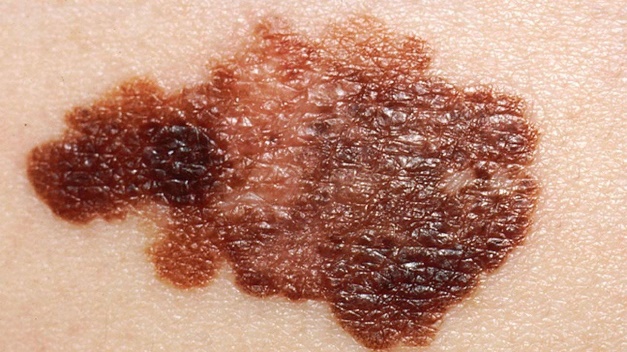
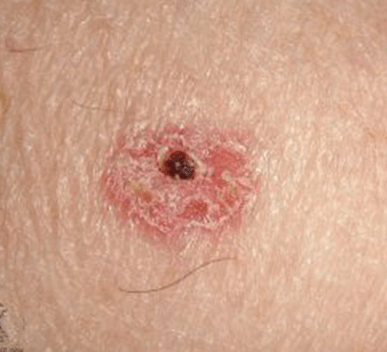
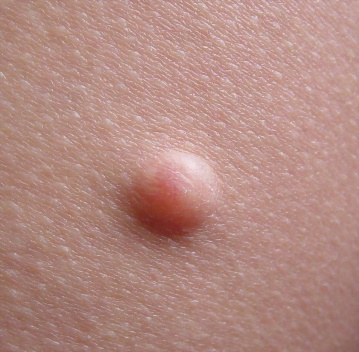
In some instances, asking a directed question can bias the participant to provide the correct answer. In these cases, less specific wording will be used for the initial question to not influence the participant. Only if the participant's knowledge, or lack thereof, cannot be sufficiently assessed through their response to the initial question, optional and more focused follow up questions will be asked. These optional, open-ended, naturally worded follow-up questions will be asked in a tiered approach.
Participants will be free to refer to any materials when answering but will not be specifically guided to any aspect of the product interface (e.g., the Electronic IFU). The study moderator will note participants' answers and record any use problems. There will then be another post scenario interview, beginning with participants being asked how they felt answering the questions and whether there was anything they identified as unclear or confusing when answering. This will again be followed by any clarifying questions and a root cause interview on all remaining use problems to evaluate safe and effective use.
| Use Scenario | Phase | Task | Classification | Success Criteria |
|---|---|---|---|---|
| HCP Use Scenario 1: Simulated Use: No Lesion | Authenticate in the system | User logs into the system. | Critical | The user enters the correct username and password and logs in successfully |
| HCP Use Scenario 1: Simulated Use: No Lesion | Authenticate in the system | User logs into the system. | Critical | The user enters the correct username and password and logs in successfully |
| HCP Use Scenario 1: Simulated Use: No Lesion | Take pictures | User takes a photo of the patient's lesion. | Critical | The photo has good quality, captures the relevant skin structure, is well lit, focused, with adequate distance |
| HCP Use Scenario 1: Simulated Use: No Lesion | Upload pictures | User uploads photos to the Legit.Health plus client. | Critical | The user uploads a high-quality image |
| HCP Use Scenario 2: Simulated Use: Lesion | Authenticate in the system | User logs into the system. | Critical | The user enters the correct username and password and logs in successfully |
| HCP Use Scenario 2: Simulated Use: Lesion | Upload pictures | User uploads photos to the Legit.Health plus client. | Critical | The user uploads a high-quality image |
| HCP Use Scenario 2: Simulated Use: Lesion | Upload pictures | User uploads photos to the Legit.Health plus client. | Critical | The user uploads a high-quality image |
| HCP Use Scenario 3: Knowledge Assessment | Read and interpret the report | The user understands that the report is not a certain diagnosis. | Critical | The user acknowledges that the output report is not a standalone diagnosis. |
| HCP Use Scenario 3: Knowledge Assessment | Read and interpret the report | User correctly interprets the report output. | Critical | The user correctly identifies the quantification of disease intensity, extent, or count of clinical signs. |
| HCP Use Scenario 3: Knowledge Assessment | Read and interpret the report | User correctly reports the probability of certain conditions based on the ICD categories. | Critical | The user identifies the probability of conditions listed in the report |
| HCP Use Scenario 3: Knowledge Assessment | Read and interpret the report | User understands the format of the device's output | Critical | The user identifies the information in the report |
Justification for “If Applicable” Use Scenarios
Some use scenarios are marked as “if applicable”. This designation does not indicate that these tasks are less important, but rather that the user behaviors, actions, and potential use errors they are intended to evaluate are already observed and assessed within other core scenarios.
In accordance with IEC 62366-1 and MDR Annex I, it is not required to obtain distinct or standalone results for each scenario when the related use behaviors are sufficiently captured elsewhere in the study. Instead, the findings from these “if applicable” scenarios are integrated into the overall analysis and results of the main simulated-use scenarios.
For example:
- ITPScenario2 – Error Handling and Recovery is optional because the integrator’s ability to interpret and respond to API errors is already assessed through error-handling tasks embedded in ITPScenario1 – Simulated Use.
- HCPScenario4 – Error Handling is optional because the healthcare professional’s ability to recognize and respond to system errors is already assessed within HCPScenario1 to HCPScenario3 – Simulated Use and Knowledge Assessment.
This approach avoids unnecessary duplication while maintaining full coverage of all use-related hazards and critical tasks. The evaluation of error recognition and recovery behaviors is therefore included within the results and observations of the primary scenarios, ensuring comprehensive evidence of safe and effective use.
Note: Even when marked as optional, all related tasks and potential use errors are analyzed and documented to ensure compliance with IEC 62366-1 §5.4 and §5.9 and MDR Annex I §14.2, §17.2, and §23.1.
Description of Data Collection and Methods
The data to be collected are as follows:
- Observational Data: Participants will be given an opportunity to use the devices independently and in as realistic a manner as possible, without guidance, coaching, praise, or critique from the study moderator.
- Interview Data: The study moderator will ask participants open-ended, neutrally-worded questions to understand any use problems participants may have with the study devices and tasks. Particular care will be taken not to blame participants or bias them in later tasks.
- Subjective assessment of use: This data consists of participants' subjective feedback on the root cause of potentially safety-related use errors and use difficulties, and opinions on all tasks for any residual risk, as well as the adequacy of instructions.
- Knowledge Task Data: Participants will be given an opportunity to explain their understanding of product use independently. This type of data is necessary as some critical tasks cannot be directly observed through simulated use; these critical tasks may involve users' understanding of information (e.g., warnings).
The following coding system will be used for simulated-use and knowledge task data collection and analysis:
- Success (OK): This coding describes a situation in which the participant correctly performs a task, or answers a knowledge task question, according to the steps in the User Guide or an acceptable alternative.
- Use error (UE): This coding describes a situation in which the participant performs a task incorrectly (i.e., not in accordance with the intended manner of use), does not complete a necessary task, or answers a knowledge task question incorrectly or incompletely.
- Close call (CC): This coding describes a situation in which the participant performs or nearly performs a task incorrectly (i.e., not in accordance with the intended manner of use), or answers a knowledge task question incorrectly or incompletely, but then resolves the issue prior to any harm being done without assistance.
- Use difficulty (UD): This coding describes a situation in which the participant struggles to some extent while completing a task or answering a knowledge task question (e.g., confusion, taking longer than expected, difficulty manipulating a device's components), but eventually can completes the task or answers the question successfully without assistance.
Recording Equipment
Audio and video will be recorded for all study sessions using synchronized, multi-channel, HD-resolution video.
Recording Software
Study data will be recorded by the study team into OpenClinica. OpenClinica is a web-based platform that allows the study moderator to directly record their scores, observations, and notes into an electronic version of the study protocol. All data entered into OpenClinica and any subsequent changes made by the study team are visible via an audit log. Any changes made to the data after it is entered requires a rationale for the change to be documented.
OpenClinica is compliant with the FDA's Title 21 CFR Part 11.
Data Analysis
Observational data during simulated-use and knowledge task data will be collected and any use problems will be recorded as moderator observations. Based on this, as previously described, an interview will be conducted to better discern any use problems (i.e., UEs, CCs, and UDs), the root cause of such problems, and the associated residual risk. Such interview data will be recorded as participant comments.
Description of Root Cause Analysis
Root cause analysis (RCA) will involve examining the data to determine the underlying cause of each use problem, with a focus on investigating contributing factors to each problem and determining ways to prevent them in the future. Moderator observations and participant comments will be analyzed holistically during this process to evaluate the safety and effectiveness of product use.
Description of Residual Risk Analysis
All risks that remain after human factors validation testing will be analyzed to determine whether they can be reduced or eliminated. This analysis will determine whether design modifications are needed, would be possible and might be effective at reducing the associated risks to acceptable levels. This analysis will include a risk-based discussion of the observed use events and their root causes as well as the effectiveness of current risk mitigations.
If user interface design modifications are required to further mitigate risk, the residual risk analysis will include a description of the recommended design changes and a discussion regarding whether additional HF validation testing is required.
The residual risk analysis will be used to evaluate the safety and effectiveness of product use and determine if the residual risk associated with product use is acceptable. While it is rarely feasible to eliminate all risks, it should be reduced to an acceptable level, where feasible and practical, such that the benefits of product use outweigh any residual risks.
Appendix A: Interview Guide
Thanks for coming in today. In a few moments, we'll start the session, but before we begin, I want to review a few things with you. You have already signed the informed consent. Do you have any additional questions about the document? I would also like to make sure you:
- Promise not to tell anyone about what you see today.
- Give us permission to audio and video record this session, so long as none of the footage is ever made public.
- Agree that the study's Sponsor can make use of any information you provide during the study without any restrictions.
[Answer any questions and then proceed.]
Ok, I'm going to turn on the video and audio recording now.
Testing Session
Today, I am going to ask you to go through a series of use scenarios with a software-only device that analyzes images of visible skin structure abnormalities to support the assessment of skin conditions. I want to get your feedback on the device and will also ask you some questions regarding the use of the device.
For the purpose of our study, we are simulating a real use scenario, so please imagine that you are in a real situation and perform all tasks as if you were at work.
If for some reason you feel that you are unable to complete a task the way you would at work due to this simulated environment, please let me know.
At times, you may have questions for me that I may or may not be able to answer. If I am unable to answer, it is because I want to observe how you would handle a real-life situation when problems arise.
Please remember that you are here to help us evaluate the product. We are not evaluating you or your abilities in any way. You are the expert here to help us improve the product today.
Finally, please keep in mind, I did not design anything you're seeing today, so you do not have to worry about my feelings regardless of whether your feedback is positive or negative. We want your honest opinions, good or bad. So, if you see any problems with the devices I will be showing you that you think I should know about, please mention them.
Do you have any questions before we start?
ITP Use Scenario 1: Simulated Use
Moderator to prepare computer with hospital EHR system installed, and link to electronic IFU.
For our first task, I would like you to imagine that your hospital is looking to incorporate the device into their system. I would like you to complete all the steps necessary to install and set up the device for use. You can refer to any of the materials provided at any time.
When you're ready, please begin and take your time.
To see the observation form, please refer to T-TF-025-005 Summative evaluation observation form
Post-Scenario Interview
How did that go?
Did you make any mistakes or almost make any mistakes during that scenario?
Did you have any difficulty at any point during that scenario?
[Follow up on participant-observed use problems and root-causes.]
[Follow up on study moderator-observed use problems and root-causes.]
HCP Use Scenario 1: Simulated Use: No Lesion
Moderator to prepare smartphone, computer with hospital EHR/EMR system installed, the device installed, and manikin with simulated skin lesions
For our first task, I would like you to imagine that an IT professional has implemented the device into your hospital EHR system. Your patient has visited your clinic because of concerns over the skin of the back of their hand (Moderator to direct participant to a researcher to take a photo of their hand). I would like you to complete all the steps necessary to assess the area by generating a report using the device. You can refer to any of the materials provided at any time.
When you're ready, please begin and take your time.
To see the observation form, please refer to T-TF-025-005 Summative evaluation observation form
Post-Scenario Interview
How did that go?
Did you make any mistakes or almost make any mistakes during that scenario?
Did you have any difficulty at any point during that scenario?
[Follow up on participant-observed use problems and root-causes.]
[Follow up on study moderator-observed use problems and root-causes after Scenario 3.]
HCP Use Scenario 2: Simulated Use: Lesion
[Moderator to prepare smartphone, computer with hospital EHR/EMR system installed, the device installed, and manikin with simulated skin lesions]
For our next task, I would like you to imagine that your patient has visited your clinic again because of concerns over a skin lesion that has appeared on their body. Imagine that these are the images you have taken of the lesion (Moderator to provide participant with images of the skin lesions provided by the sponsor) I would like you to complete all the steps necessary to assess the lesion by generating a report using the device. You can refer to any of the materials provided at any time.
When you're ready, please begin and take your time.
To see the observation form, please refer to T-TF-025-005 Summative evaluation observation form
Post-Scenario Interview
How did that go?
Did you make any mistakes or almost make any mistakes during that scenario?
Did you have any difficulty at any point during that scenario?
[Follow up on participant-observed use problems and root-causes.]
[Follow up on study moderator-observed use problems and root-causes after Scenario 3.]
HCP Use Scenario 3: Knowledge Assessment
Now I'm going to ask you a few questions about the device user interface. Also, this is not a test of any kind; you may refer to any of the materials provided at any point to help you answer the questions.
To see the questionnaire, please refer to T-TF-025-006 Summative evaluation questionnaire
Post-Scenario Interview
How was answering those questions?
Did you notice anything related to the product and its associated materials that was unclear or confusing when answering those questions?
Follow up on all (study moderator-observed and participant-observed) use problems and root-causes up to this point (that is, from Scenario 3).
Thank and Exit
Do you have any additional questions or comments about anything that you have seen or done here today?
That is the end of our session. Thank you for your time and feedback. [Stop recording and walk participant out.]
Were there any protocol deviations?
If so, record:
- Yes
- No
Appendix B: Electronic IFU
Electronic IFU is available at the following URL: https://apidocs-draft.legit.health.
Appendix C: List of participants recruited for summative evaluation
| ID | Nationality | User group | Sex | Age | Profession | Years of experience |
|---|---|---|---|---|---|---|
| ITP-001 | EU | ITP | F | 32 | IT Professional | 5 |
| ITP-002 | EU | ITP | F | 29 | IT Professional | 3 |
| ITP-003 | EU | ITP | F | 45 | IT Professional | 7 |
| ITP-004 | EU | ITP | M | 38 | IT Professional | 10 |
| ITP-005 | EU | ITP | M | 28 | IT Professional | 3 |
| ITP-006 | EU | ITP | M | 37 | IT Professional | 7 |
| ITP-007 | EU | ITP | M | 28 | IT Professional | 10 |
| ITP-008 | EU | ITP | M | 35 | IT Professional | 4 |
| ITP-009 | EU | ITP | M | 36 | IT Professional | 10 |
| ITP-010 | EU | ITP | M | 38 | IT Professional | 10 |
| HCP-001 | EU | HCP | M | 45 | Primary care practitioner | 3 |
| HCP-001 | EU | HCP | M | 32 | Primary care practitioner | 3 |
| HCP-001 | EU | HCP | F | 38 | Primary care practitioner | 3 |
| HCP-001 | EU | HCP | F | 47 | Primary care practitioner | 3 |
| HCP-001 | EU | HCP | F | 54 | Primary care practitioner | 3 |
| HCP-002 | EU | HCP | M | 29 | Dermatologist | 2 |
| HCP-002 | EU | HCP | F | 43 | Dermatologist | 12 |
| HCP-002 | EU | HCP | F | 52 | Dermatologist | 18 |
| HCP-003 | EU | HCP | M | 45 | Nurse | 10 |
| HCP-003 | EU | HCP | M | 45 | Nurse | 10 |
| HCP-003 | EU | HCP | M | 45 | Nurse | 10 |
| HCP-003 | EU | HCP | M | 45 | Nurse | 10 |
| HCP-003 | EU | HCP | F | 45 | Nurse | 10 |
| HCP-003 | EU | HCP | F | 45 | Nurse | 10 |
| HCP-003 | EU | HCP | F | 45 | Nurse | 10 |
| ITP-101 | US | ITP | M | 32 | IT Professional | 5 |
| ITP-102 | US | ITP | F | 29 | IT Professional | 3 |
| ITP-103 | US | ITP | M | 45 | IT Professional | 15 |
| HCP-101 | US | HCP | M | 32 | Primary care practitioner | 5 |
| HCP-102 | US | HCP | F | 29 | Dermatologist | 3 |
| HCP-103 | US | HCP | M | 45 | Nurse | 8 |