R-012-010 Device backup verification_2024_001
Evidence
Date
20241112 (YYYYMMDD)
Responsible
JD-005 Alfonso Medela
Evidence
Environment Setup
To ensure a reproducible test environment:
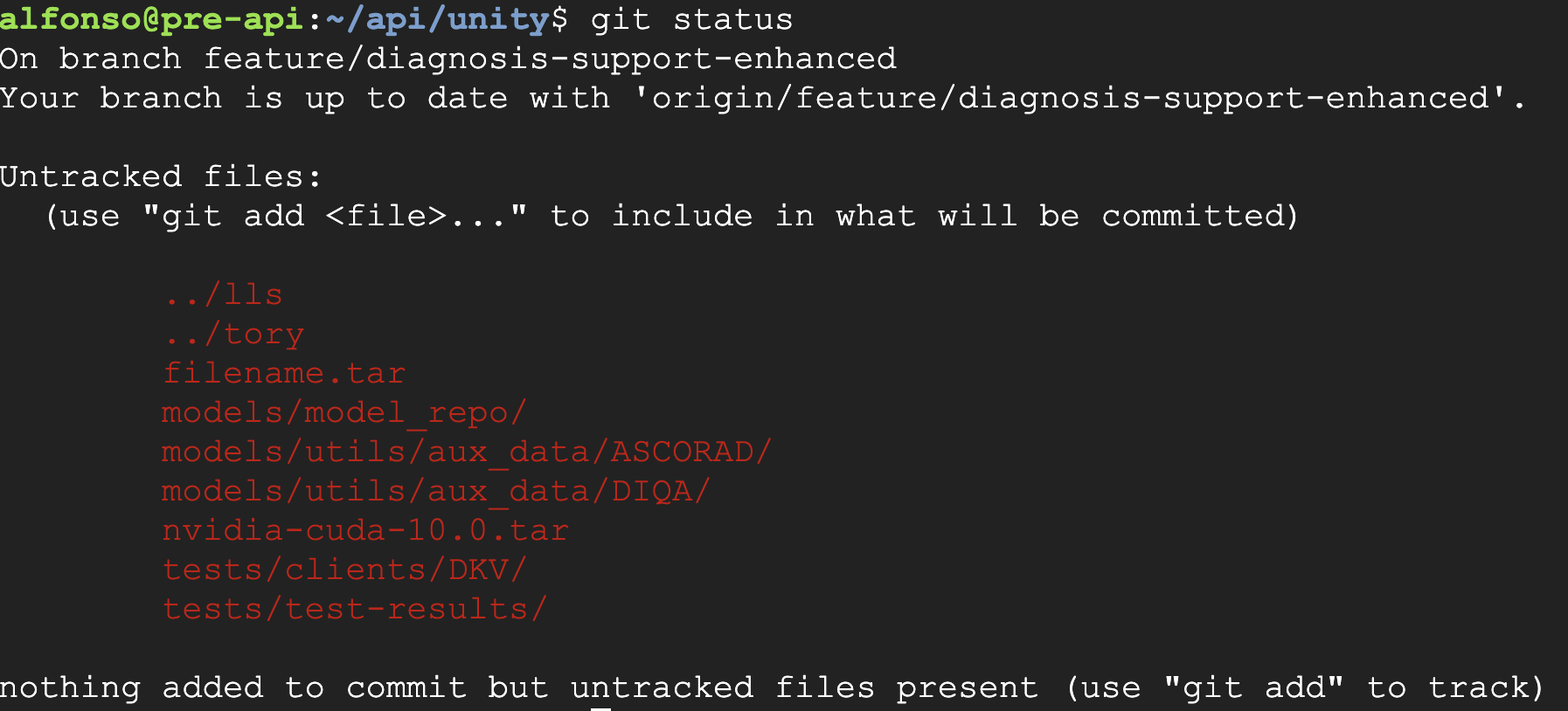
- A clean working directory was established to prevent conflicts.
- The git repository was inspected to confirm the starting state.
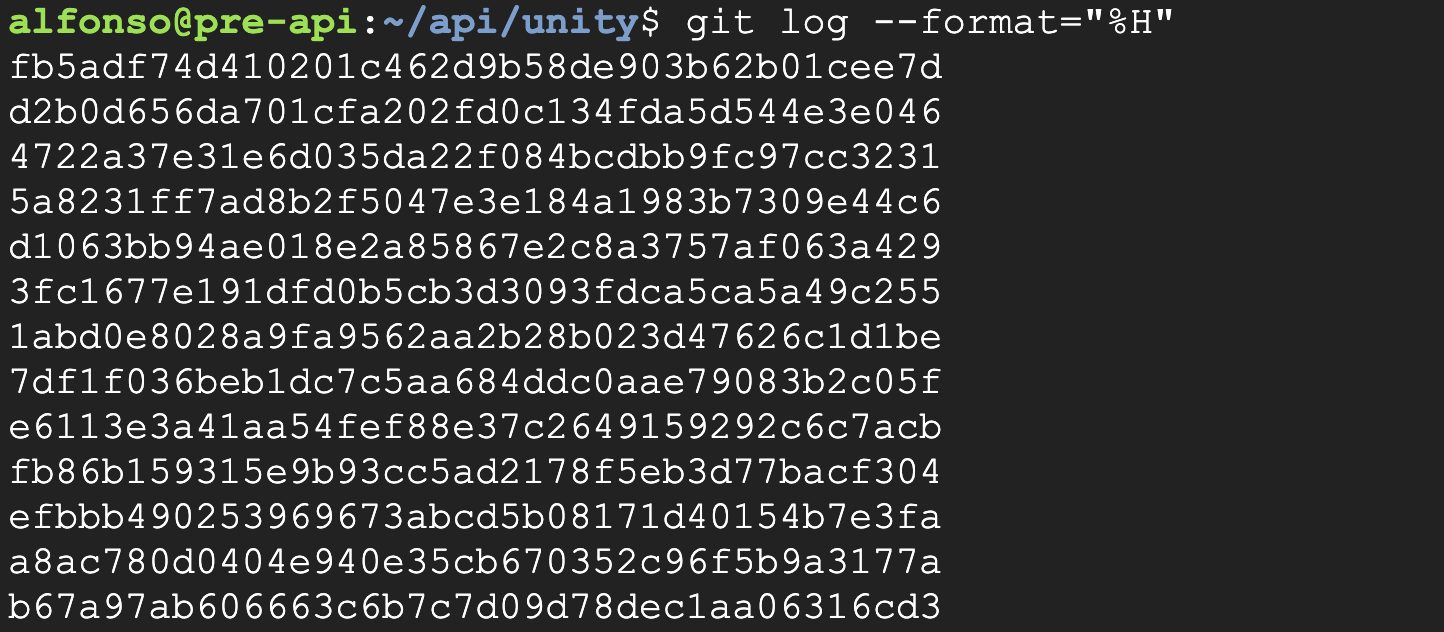
Repository Reversion
The repository was reverted to the previous commit (SHA: d2b0d656da701cfa202fd0c134fda5d544e3e046) to validate the backup state.
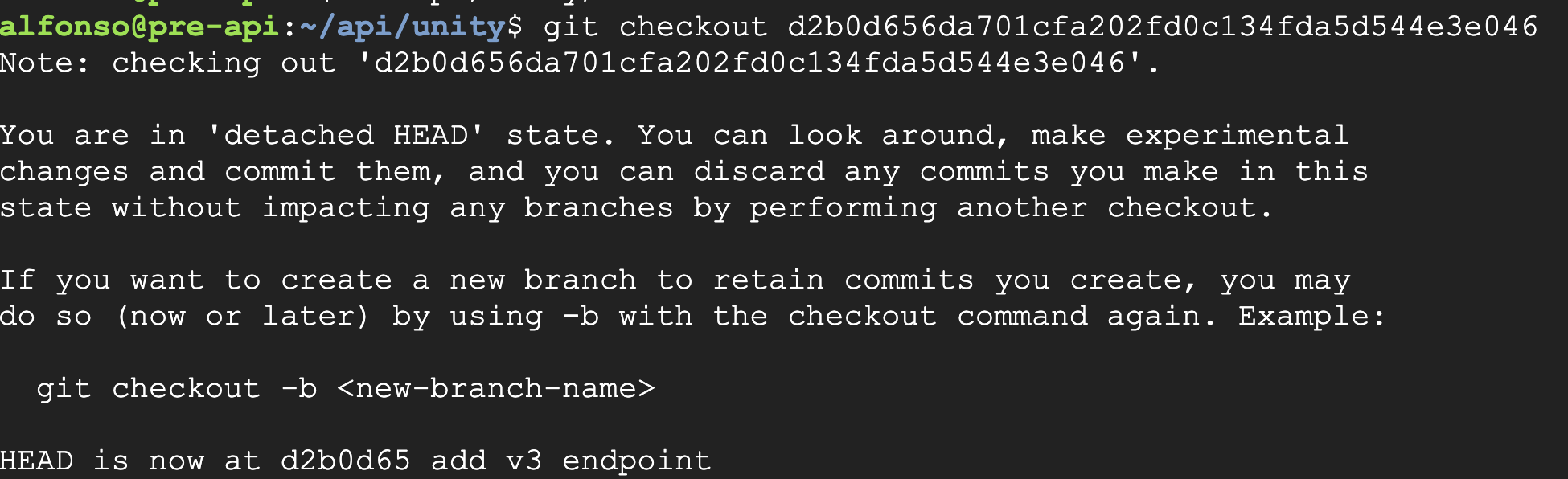
The repository was then restored to the latest commit to proceed with the build process.
Device Compilation
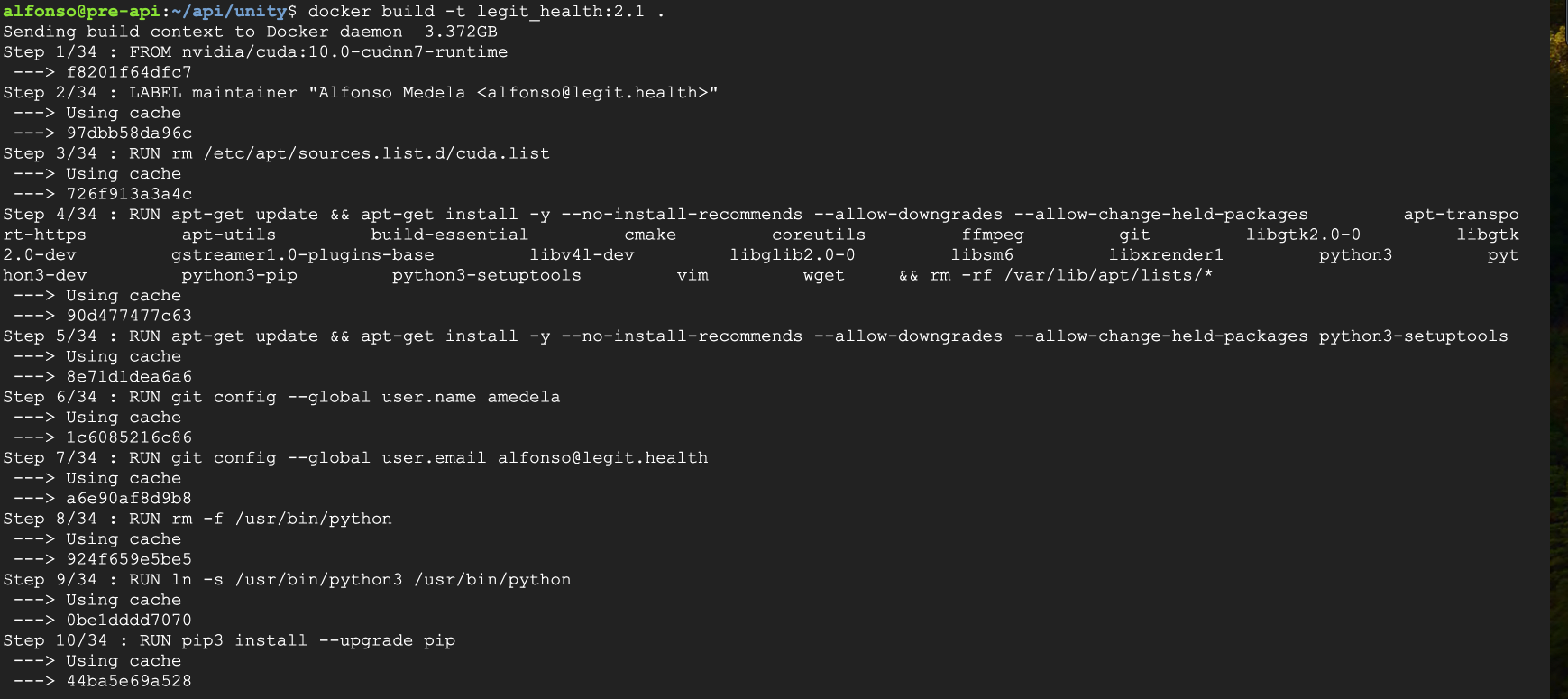
The medical device, configured as a Dockerized application, was compiled using the appropriate Docker build command.
Device Deployment and Validation
The compiled device was launched, and connectivity was verified through the designated server port.

The device responded as expected, confirming the backup and restoration processes were successful.
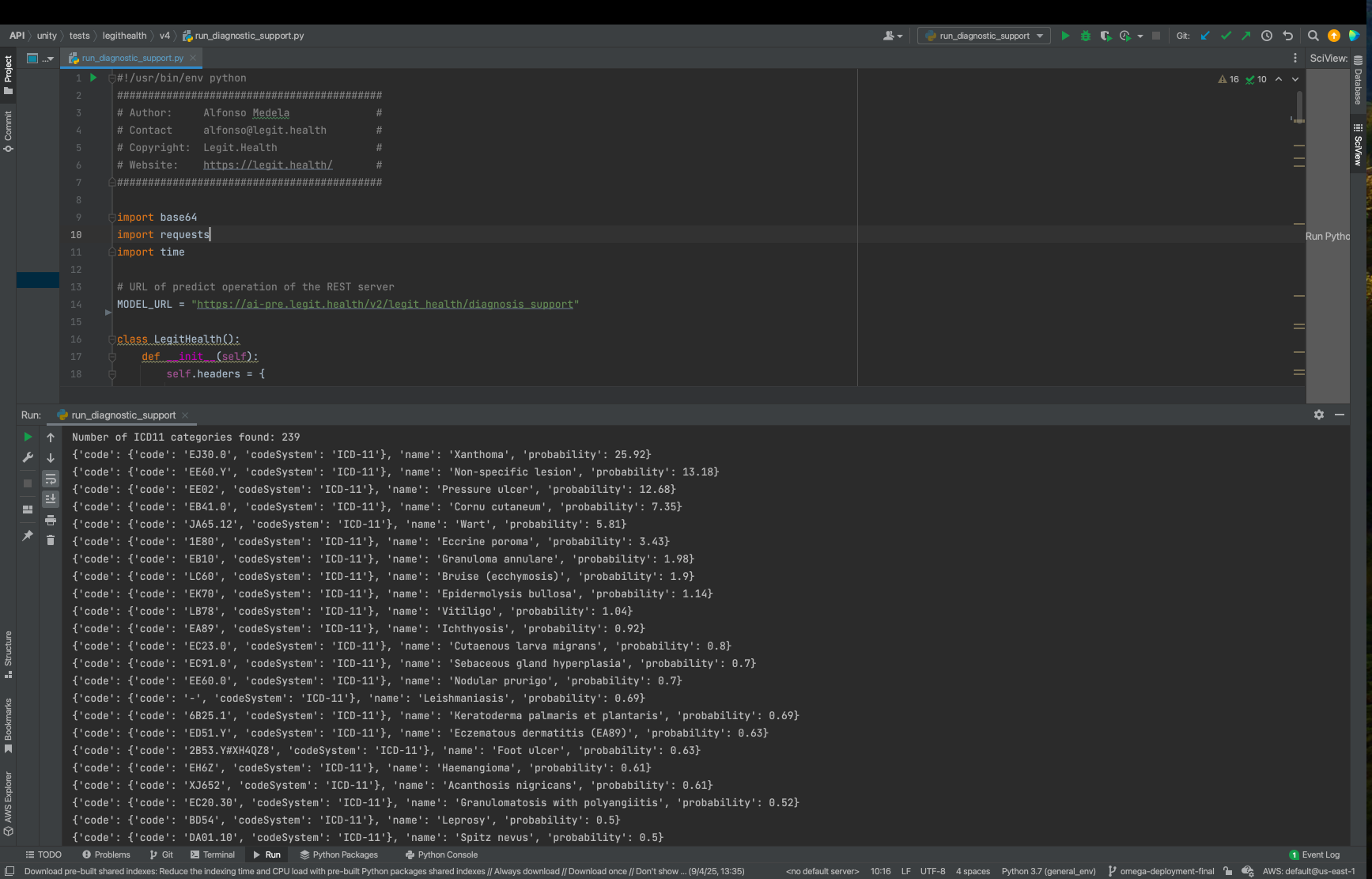
Conclusion
The backup verification test was completed successfully, with the medical device demonstrating the expected behavior post-reversion and recompilation. All steps adhered to procedure GP-012.
Signature meaning
The signatures for the approval process of this document can be found in the verified commits at the repository for the QMS. As a reference, the team members who are expected to participate in this document and their roles in the approval process, as defined in Annex I Responsibility Matrix of the GP-001, are:
- Author: Team members involved
- Reviewer: JD-003 Design & Development Manager, JD-004 Quality Manager & PRRC
- Approver: JD-001 General Manager