TEST_010 The user specifies the body site of the skin structure
Test type
System
Linked activities
- MDS-453
Result
- Passed
- Failed
Description
Tests carried out at the receiver component, to verify that users can specify the body site of the skin structure.
Objective
The objective is to enable users to submit information about the body site of the skin structure, thereby offering supplementary and valuable data for the clinical algorithm's analysis.
Acceptance criteria
Medical device accepts body sites in the request.
Materials & methods
First, we established a taxonomy for encoding within the medical device.
HEAD_TOP
HEAD_FRONT
HEAD_BACK
HEAD_LEFT
HEAD_RIGHT
ARM_LEFT
ARM_RIGHT
TRUNK_FRONT
TRUNK_BACK
LEG_LEFT
LEG_RIGHT
HAND_LEFT
HAND_RIGHT
FOOT_LEFT
FOOT_RIGHT
GENITAL
SCALP
EAR_LEFT
EAR_RIGHT
PERIORAL
TONGUE
ELBOW_LEFT
ELBOW_RIGHT
UPPER_ARM_LEFT
UPPER_ARM_RIGHT
LOWER_ARM_LEFT
LOWER_ARM_RIGHT
KNEE_LEFT
KNEE_RIGHT
UPPER_LEG_LEFT
UPPER_LEG_RIGHT
LOWER_LEG_LEFT
LOWER_LEG_RIGHT
Subsequently, we devised a method that adheres to the FHIR standard, ensuring user-friendly interaction with the device. Users can incorporate the body site information under the bodySite key within the payload.
Results
The receiver accurately handles body site data and transmits it to the orchestrator.
Protocol deviations
There were no deviations from initial protocol.
Conclusions
Users can interact with the device by submitting the body site where the lesion is located.
Test checklist
The following checklist verifies the completion of the goals and metrics specified in the requirement https://legithealth.atlassian.nethttps://legithealth.atlassian.net/wiki/spaces/LPDHF/pages/1219395593/REQ_011+The+user+specifies+the+body+site+of+the+skin+structure .
Requirement verification
- Medical device accepts body sites in the request
Evidence
The following request includes the HEAD_BACK body site and the output score is 10.5.
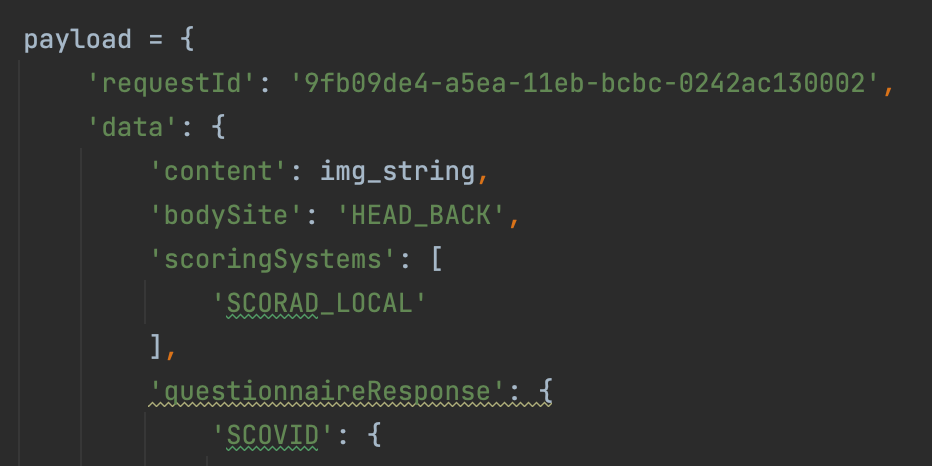
A different result is obtained if we send the same image with the TRUNK_FRONT body site:
Signature meaning
The signatures for the approval process of this document can be found in the verified commits at the repository for the QMS. As a reference, the team members who are expected to participate in this document and their roles in the approval process, as defined in Annex I Responsibility Matrix of the GP-001, are:
- Tester: JD-017, JD-009, JD-004
- Approver: JD-005